Official Journals By StatPerson Publication
|
Table of Content - Volume 10 Issue 2 - May 2019
Association of serum leptin and diastolic blood pressure in pre-eclamptic women
N Dheebalakshmi1, V Rajagopal2*
1Associate Professor, Department of Biochemistry, Govt. Coimbatore Medical College, Coimbatore – 14, Tamil Nadu, INDIA. 2Assistant Professor, Department of Biochemistry, Madurai Medical College, Madurai-20, Tamil Nadu, INDIA. Email: dr.rajgo@gmail.com
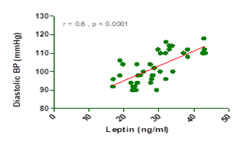
Abstract Background: Maternal peripheral leptin levels are enhanced during normal pregnancy and collectively suggest that leptin concentrations peak in the second trimester. A better understanding of leptin and its role in PE may eventually impact those causes of human perinatal morbidity and mortality. Aim: To find out the association of serum leptin and diastolic blood pressure in pre-eclamptic women. Material and Methods: This hospital-based case control study involved 40 normal pregnant and 40 pre-eclamptic women. Serum leptin was estimated by Enzyme immunoassay for quantitative determination of Human Leptin in serum. The serum leptin levels were correlated with diastolic blood pressure. Results: Mean serum leptin concentration was significantly higher in PE women compared to controls 28.85±7.2 ng/ml vs. 24.80±6.5 ng/ml. A significant positive correlation (r =0.6, p <0.0001) was observed between serum leptin and diastolic blood pressure in pre-eclampstic women. Conclusion: Leptin levels were significantly elevated in PE than in normal pregnant women. Serum leptin was also positively correlated with blood pressure. Key Word: Preeclampsia, serum leptin, diastolic blood pressure, association.
INTRODUCTION Pre-eclampsia (PE) is responsible for 50-70% cases of hypertension in pregnancy.1 It is a severe complication of human pregnancy with a worldwide incidence of 3-5%.2 It is one of the leading causes of maternal, as well as perinatal morbidity and mortality, even in developed countries.3 The abnormalities in the development of placental vasculature early in pregnancy resulting in relative placental ischemia, leads to release of factors into the maternal circulation. These factors alter maternal systemic endothelial function and cause hypertension and other manifestations of the disease.4 Leptin, a circulating placental protein, has also been implicated in the pathogenesis of many of the maternal features of the disease.5 Leptin plays role in normal pregnancy which include the regulation of conceptus growth and development, fetal/placental angiogenesis, embryonic hematopoiesis, and hormone biosynthesis within the maternal-fetoplacental unit. Numerous studies have demonstrated that maternal peripheral leptin levels are enhanced during normal pregnancy and collectively suggest that leptin concentrations peak in the second trimester.6,7 A better understanding of leptin and its role(s) in PE may eventually impact those causes of human perinatal morbidity and mortality. The present study was conducted to find out the association of serum leptin and diastolic blood pressure in pre-eclamptic women.
MATERIAL AND METHODS This hospital based case control study involved 40 normal pregnant and 40 pre-eclamptic women over a period of two years in the Department of Obstetrics and Gynaecology. The study subjects were divided in two groups: Group A (Preeclamptic women) and Group B (normal pregnant women) matched for age and gestational age. Inclusion criteria: Group A consisted of 40 obstetric patients, diagnosed as having pre-eclampsia according to ISSHP (International society for the study of hypertension). Pre-eclamptic women were then divided according to the severity of the disease into mild preeclampsia (BP ≥140/90 -159/110 mm Hg, and proteinuria ≥ 0.3-5.0 g/day) and severe preeclampsia (BP ≥ 160/110 mm Hg and proteinuria ≥ 5.0 g/day). Group B consisted of 40 healthy pregnant subjects having blood pressure <120/80mmHg and no significant proteinuria, without any previous history of hospitalization or any medical complication and were considered as controls. Exclusion criteria: Pregnant women with Multifetal gestation, chronic hypertension, diabetes mellitus, autoimmune diseases, vascular diseases, renal disorder, maternal or fetal infection and fetal congenital anomaly were excluded from the study. The Institutional Ethical and Research Committee approved the study protocol and informed consent was obtained from the controls and the patients before the collection of the blood samples. At the time of registration, a detailed obstetric and medical history was taken by following a pre-designed data sheet. General, systemic and obstetric examinations were carried out on the same day. Measurement of BP: The arterial blood pressure in the brachial artery was measured by using a simple mercury sphygmomanometer on right arm in a comfortable sitting position after 10 minutes of rest. Blood pressure was measured using both palpatory and auscultatory methods. The reported values represent the mean of two readings taken at 5 minutes interval. Estimation of serum leptin: Serum leptin was estimated by Enzyme immunoassay for quantitative determination of Human LEPTIN in serum. The DRG Leptin ELISA Kit is a solid phase enzyme-linked immunosorbent assay (ELISA) based on the sandwich principle. The microtiter wells are coated with a monoclonal antibody directed towards a unique antigenic site on a Leptin molecule. An aliquot of patient sample containing endogenous Leptin was incubated in the coated well with a specific rabbit anti Leptin antibody. A sandwich complex is formed. After incubation the unbound material was washed off and an anti-rabbit peroxidase conjugate was added for detection of the bound Leptin. Having added the substrate solution, the intensity of colour developed was proportionate to the concentration of Leptin in the patient sample. Statistical analysis: Data analysis was done on computer package SPSS (Statistical Package for Social Sciences). The Statistical significance of difference between the mean values of two groups was evaluated by the student's "t" test. The values were expressed as mean ± standard deviation. The difference in the mean values of the two groups was regarded as statistically significant, if the p-Value was less than 0.05 and it was taken as highly significant, if p-Value was less than 0.001. Correlation Coefficient was detected using Pearson Coefficient of Correlation. For data feeding, the computer package Microsoft Excel was used. RESULTS The study included 80 pregnant women. Of these 40 women had uncomplicated pregnancies (Group B) and 40 were diagnosed as PE (Group A). The pre-eclamptic group was again divided according to the severity of the disease into patients with mild PE (n=23) and patients with severe PE (n=17). Table 1: Anthropometric and clinical parameters of the study subjects
The two study groups were statistically similar in age and gestational age. As expected from the recruitment criteria, the preeclampsia group had significantly higher systolic and diastolic BP values (p<0.0001). Mean serum leptin concentration was significantly higher in PE women compared to controls 28.85±7.2 ng/ml vs. 24.80±6.5 ng/ml, p<0.01.
Figure 1: Correlation between leptin and diastolic BP in pre-eclampsia DISCUSSION Preeclampsia is a disease that begins in the placenta and ends at the maternal endothelium and is a major cause of maternal morbidity and mortality in the developed countries.3 It is significantly associated with alterations of maternal physiologic characteristics and metabolism manifesting itself primarily as hypertension with arteriolar vasoconstriction. The two study groups were statistically similar in age and gestational age as shown in Table 1. The mean systolic and diastolic blood pressure was higher in PE women than in normal pregnant women (p<0.001). Contrast to the present study few investigators have concluded that maternal serum leptin levels are reduced in preeclampsia,6,7 and in another study,8 the development of preeclampsia has been associated with reduced plasma leptin in the second trimester. plasma leptin levels in the severe PE group were significantly higher than those in the mild PE group. In severe PE, placental production of leptin is increased in response to hypoxia, thereby supporting the notion that augmented plasma leptin levels in severe PE reflect placental hypoperfusion and/or hypoxia. Because hypoxia induces a set of several placental genes in trophoblast,9 augmented placental production of leptin may represent one of the generalized hypoxic responses of trophoblasts in PE. These findings were also supported by Hauguel-de et al10 who showed that placental leptin production is increased in choriocarcinoma, preeclampsia and type 1 diabetes. Estrogens, hypoxia and insulin have been suggested as positive regulators of placental leptin production. Therefore, leptin may serve as a placenta-derived marker of PE, possibly reflecting placental hypoxia associated with severe PE. There was also a positive correlation between serum leptin and diastolic blood pressure values as observed in Fig 1. It has been shown that intra-cerebroventricular leptin infusion increases arterial pressure, indicating its important role in development of hypertension.11 Another theory has also been proposed that Leptin causes an increase in noradrenaline turnover to the brown adipose tissue,12 thereby increasing the sympathetic outflow in PE.13
CONCLUSION From this comparative case control study, it was observed that Leptin levels were significantly elevated in PE than in normal pregnant women. Serum leptin was also positively correlated with blood pressure. Thus, serum leptin level was increased with the severity of pre-eclampsia.
REFERENCES
|
 Home
Home