Official Journals By StatPerson Publication
|
Table of Content - Volume 12 Issue 1 - October 2019
Study on laboratory critical value analysis in a multi-speciality hospital
Kapil Bhatia1, Pallavi Bhatia2*, Sneha N Udari3, Neelam Patil4
1Associate Professor, Department of Biochemistry, Armed Forces Medical College, Pune, Maharashtra, INDIA. 3Ex Student, Masters in Hospital Administration, Centre for Health Management Studies and Research, Bharati Vidyapeeth Deemed University, Pune, Maharashtra, INDIA. 4Associate Professor, Department of Biochemistry, TNMC and BYL Nair Ch. Hospital, Mumbai, Maharashtra, INDIA. Email: pallavikb@yahoo.co.in
Abstract Background: One of the most important functions of a clinical laboratory is the clear, accurate, and rapid communication of a critical value to patient care providers. Critical values are important and patients require immediate treatment. The present study was aimed to analyze the compliance of critical care reporting of the laboratory tests. Materials and Methods: The study was conducted in a National Accreditation Board of Hospitals (NABH) accredited multispecialty hospital. The data was collected with an observational checklist prepared according to the National Centre for Disease Control and Prevention with two options either ‘yes’ or ‘no’. The percentages were calculated in the two groups, one initially and the second after imparting the education related to the importance of the critical value generated. Results: Compliance related to critical value time of report informed was 97.22% and critical report stamp was 63.88% initially. After imparting medical education related to importance of critical value the compliance related to time of report informed was 100% and critical report stamp was 72.22%. Conclusions: Critical value reporting is an important phase of the clinical laboratory testing process. The final aim should be that these values must be with the right person, as fast as possible, so that the treating doctors are quickly notified of immediate life threatening conditions and patients receives the highest quality of care. Keywords: Clinical laboratory, Compliance, Medical education, Critical value
INTRODUCTION The importance of clinical laboratories is currently acknowledged in medical world; around 70% of decisions are taken based on laboratory investigations.1 One of the most important functions of a clinical laboratory is the clear, precise, and quick communication of a critical value to health care providers.2Critical value policies used by clinical laboratories ensure that caregivers are notified of patient’s life-threatening results. Lundberg.3 was the first to define critical values as results that may lead to adverse outcomes for patients if clinicians were not notified urgently of the critical result.Critical value reporting subsequently became an accreditation requirement, with most laboratories having implemented critical value policies as a quality assurance practice. Laboratory professionals are often challenged with many hindrances in the reporting of critical values, including establishing clinically relevant criteria for critical values, resolving difficulties in finding the doctor who ordered the report when a critical value is obtained, and confirming that the doctor understands the severity and implications of a critical result. Critical value is important and require immediate notification and immediate treatment or more intensive care.The present study was aimed to analyze the compliance of critical care reporting of the laboratory tests and to see the effect of medical education on compliance of critical care reporting. Objectives: 1) To identify the errors occurring while doing critical value documentation. 2) To analyze the response of critical value in ward in the form of critical value stamp. 3) To propose recommendation based on study findings.
MATERIALS AND METHODS The study was designed to identify areas of practice that could be improved upon in order to reduce the medication errors and improve patient safety. The prospective study was conducted at Rao Nursing Home; a 125 bedded NABH accredited multispecialty hospital in Pune. Duration of study was from 16th Feb 2017 to 30th March 2017.Quantitative data was collected and analyzed in this study for addressing the research statement in a comprehensive manner. The data for the study has been collected with an observational checklist with the options ‘yes’ or ‘no’. The Observation Checklist has been prepared by the guidelines provided by Center for Disease Control and Prevention (CDC). Observational checklist included different criteria’s for compliance of critical value reporting as under a. Diagnosis b. Name of investigation c. Date of investigation d. Reported by e. Time of report generated f. Time of report informed g. Reported to h. Critical report stamp i. Action taken on critical report j. Signature of consultant k. Appropriateness of response Data was collected initially for compliance of critical value reporting. Training sessions were organized by doctors to increase knowledge, practice and attitude of staff towards compliance of critical value reporting and the data for critical value reporting were collected after the training sessions again. The data thus generated was analyzed using percentages and was presented in tabular form and graphically.
RESULTS Initially 100 % compliance of critical value reporting was seen in diagnosis, name of investigation, date of investigation, reported by, time of report generated, reported to, action taken on critical report, signature of consultant and appropriateness of response. Compliance in relation to time of report informed is 97.22% and for critical report stamp is 63.88%. After imparting training sessions in all criteria compliance of critical value reporting was 100% except critical report stamp which was increased to 72.22%.The average time of report informed in case of critical value report is 5 minutes for the inpatients. The laboratory is using telephonic calls to give the critical value report to the ordering doctor. To verify the accuracy of patient information communicated via the telephone, the doctor responsible for patient care do the read-back of unique patient numeric identification number, patients name and critical test result(s). The details of the result are shown in Table 1 and Table 2 and Graph 1 and Graph 2 as under. Table 1: Percentage Compliance of Critical Value Reporting before the training sessions
Table 2: Percentage Compliance of Critical Value Reporting after the training sessions
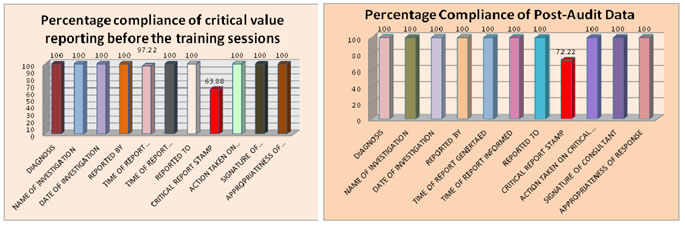
DISCUSSION A critical value is most often a numerical determination of a physiologic abnormality. The numerical value may be determined to be abnormal by variance from a mean value and is related with evidence-based risk to a patient's likelihood of poor outcome unless clinical intervention is given priority.4 Although most clinical laboratory results have diagnostic and therapeutic implications that do not require immediate doctor involvement, however, laboratory findings are sometimes much altered, and may indicate a potentially fatal outcome to the patient.In our study most of the criteria’s for critical value reporting were full filledinitially itself except the time of report informed which has the compliance of 97.22% and critical report stamp which has the compliance of 63.88%. Such high compliance can be because the hospital is NABH accredited. After the training sessions highlighting the importance of critical value report and its impact on patient safety taken by the doctors the compliance of all criteria’s was 100% except that of critical report stamp which increased to 72.22%; it shows that training of the staff reduces the number of critical reporting errors and these errors can further be reduced by conducting training sessions by doctors on regular basis. In our study it was found that the time spend to inform the critical value report for the inpatients on the average was 5 minute. This is in accordance with the study conducted by Howanitz et al5 which states that the mean time spent communicating a single critical-value result is 6 minutes for inpatients and is also in agreement of the study conducted by Yagan D et al6 which states that laboratory personnel need 4-6 minutes to complete a critical value call for hospital inpatients. In our study the critical value reports were informed by laboratory doctor or senior laboratory technician to the concerned doctor or the care giver if the doctor is not available on telephone. This is in accordance with the US healthcare institutions(90%)where the medical technologist performing the test are responsible for communicating critical values5,7and also in Italy8where senior laboratory staff takes the responsibility at most(81%) hospitals. The limitations of this study are only critical value report of inpatients were taken into account and that also for short duration of the study. The value of the study can be further enhanced if critical value report of outpatients is also included and also by increasing the duration of the study. Furthermore the critical value reports in the hospital are informed by the most common traditional practice of telephonic system which could be improved and there could be upgraded electronic system, which helps to inform critical value reports directly to the respective ward/ICU setting by checking to patient’s unique identification number. From our study it is recommended that patient’s case sheets should be regularly checked and monitored for the critical report stamp. Also the frequency of training sessions by doctors should be increased for further decreasing the critical value criteria errors and bringing the favorable patient outcome.
CONCLUSION Critical value reporting is an important phase of the clinical laboratory testing process and if criteria’s for critical value reporting are not adequately matched it indicates the futility of the process. The final aim should be that these values must be with the right person, as fast as possible, so that the doctors give immediate treatment to the patient’s and improve the final outcome of the patient’s.
REFERENCES
|
 Home
Home
