Official Journals By StatPerson Publication
|
Table of Content - Volume 12 Issue 1 - October 2019
Cardioprotective efficacy of the active petroleum ether fraction of Gardenia gummifera Linn.f. on isoproterenol induced myocardial infarction in rats
S P Prabha1, M S Latha2, A Geetha1, C Sumina1, P N Ansil3*
1Department of Biochemistry, Pushpagiri Institute of Medical science and Research Centre, Thiruvalla, Pathanamthitta, Kerala, INDIA. 2Biochemistry and Pharmacognosy Research Laboratory, School of Biosciences, Mahatma Gandhi University, P.D. Hills. P.O, Kottayam,Kerala, INDIA. 3Assistant Professor, Department of Biochemistry, T.K.M. College of Arts and Science, TKM College P.O., Karicode, Kollam, Kerala, INDIA. Email: ansilpn@gmail.com
Abstract Background: Gardenia gummifera Linn.f. (Rubiaceae), a medicinal shrub used in Indian traditional medicine for the treatment of obesity, cardiac debility and lipolytic disorders. Oleanolic aldehyde and vernolic acid are the active components identified from the petroleum ether fraction (PEF) of Gardenia gummifera Linn.f. The present work aim to study the cardioprotective effect of PEF of Gardenia gummifera root on Isoproterenol (ISO) induced myocardial infarction (MI) in rats. Methodology: Myocardial infarction was induced by the subcutaneous injection of ISO (6mg/100g body weight) at an interval of 24h for 2 days. PEF (20 mg/kg, p.o.) was given to rats once daily for 15 days prior to the ISO challenge. The myocardial damage was assessed by quantifying the serum levels of cardiac marker enzymes (LDH, AST, ALT, CK-MB) and Cholesterol and triglyceride. The heart tissue antioxidants such as Catalase (CAT), Glutathione peroxidase (GPx), Glutathione reductase (GR), Glutathione-S-transferase (GST) and Reduced Glutathione (GSH) were altered in MI rats. The level of lipid peroxidation was measured as malondialdehyde (MDA) and quantification of histopathological changes also supported protective effects of MEGG. Results: PEF significantly (p ≤ 0.05) protected the above mentioned parameters to fall from the normal levels. Histopathological evaluation also supported the protective effects of PEF. Conclusion: Our results suggest that PEF of Gardenia gummifera Linn.f. affords protection against isoproterenol-induced myocardial infarction. Key Words: Cardioprotection; Gardenia gummifera; Isoproterenol; Myocardial infarction; ALT; AST.
INTRODUCTION Ischemic heart diseases (IHD) remain the principal cause of death in both developed and developing countries, accounting for about 20% of death per year worldwide. Experimental and clinical studies have shown that during ischemic injury, oxidative stress produced by the generation of reactive oxygen species (ROS) plays a key role in the development of MI 1. In ischemic tissues, the free radicals and ROS have been implicated in oxidative chain reactions which damage the cell membrane and subsequently, structural and metabolic alterations, leading to cardiac dysfunction and ultimately cell death 2. The use of natural antioxidants is increasing as defensive agents against number of cardiovascular abnormalities. The bioactive agents from herbal sources have gained primary importance in modern system of medicines, reducing the risks of cardiac ailments by scavenging the free radicals formation 3. Cardioprotective potential of medicinal plants can be evaluated in chemically induced myocardial infarction in animal models.
MATERIALS AND METHODS Chemicals Isoproterenol hydrochloride was purchased from Sigma chemicals (St. Louis, MO, USA). Assay kits for serum lactate dehydrogenase (LDH), serum transaminases (ALT, AST), creatine kinase isoenzyme (CK-MB), triglycerides and cholesterol were purchased from Agappe Diagnostic Ltd., India. Preparation of the active Petroleum Ether Fraction Gardenia gummifera Linn. f. were collected from its natural habitat (Idukki Dist., Kerala, India) and identified. A voucher specimen (SBSBRL.05) is maintained in School of Biosciences, M.G University, Kottayam. The shade dried roots of G. gummifera were powdered and soxhlet extracted with methanol (50 g in 400 mL) and were concentrated under reduced pressure using a rotary evaporator. The percentage yield of methanolic extract in our study was approximately 10.3% (w/w). The methanolic extract thus obtained was then taken in a round bottom flask of simple condenser and further fractionated using solvents of increasing polarity, viz. petroleum ether, chloroform, ethyl acetate and methanol. For animal experiment the active sub-fraction (PEF) was prepared as a suspension in 5% Tween 80. Animals and diets Adult male wistar rats weighing 150 ± 7.6 gm (Mean ± S.D, n = 30) were used in this study. The rats were fed with standard laboratory chow (Hindustan Lever Foods, Bangalore, India) and provided with water ad libitum. The animals were maintained at a controlled condition of temperature of 26-280C with a 12h dark cycle. Animal studies were followed according to Institute Animal Ethics Committee (IAEC) regulations approved by Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA) (Reg. No. B2442009/3) and conducted humanely. Experimental Protocol The animals were divided in to 5 groups (Six rats/group).
Twenty four hours after the second dose of ISO injection, the animals were sacrificed and the blood was collected and serum was separated by centrifugation. The heart tissue was excised immediately, washed with chilled isotonic saline, and used for antioxidant and histopathological studies. Biochemical analysis. Serum analysis Cardiotoxicity was assessed by quantifying the serum levels of AST (EC 2.6.1.1), ALT (EC 2.6.1.2), LDH (EC 1.1.1.27) CK- MB (EC 2.7.3.2), TC and TG by using the kit of Agappe Diagnostic Ltd., India. Activities of these serum enzymes were measured using semi autoanalyzer (RMS, India). Tissue analysis Heart was excised, washed thoroughly in ice-cold saline to remove the blood. 10% of homogenate was prepared in 0.1M Tris HCl buffer (pH – 7.4). The homogenate was centrifuged at 3000 rpm for 20 min at 4oC and the supernatant was used for the estimation of catalase (CAT), glutathione peroxidase (GPx), reduced glutathione (GSH), lipid peroxidation product (Thiobarbituric Acid Reactive Substances – TBARS) and total protein. Histopathological studies Small pieces of heart fixed in 10% buffered formalin were processed for embedding in paraffin. Sections of (5-6 µm) were cut and stained with haematoxylin and eosin and examined for histopathological changes under the microscope (Motic AE 21, Germany). The microphotographs were taken using Moticam 1000 camera at original magnification of 100x. Statistical Analysis Results were expressed as mean + S.D. and all statistical comparisons were made by means of one way ANOVA test followed by Tukey’s post hoc analysis and p – values less than or equal to 0.05 were considered significant. RESULTS Biochemical analysis Table 1and 2 shows the activities of serum marker enzymes such as AST, ALT, CK-MB, LDH and serum levels of total cholesterol (TC) and triglycerides (TG) in control and experimental groups of rats. Treatment with PEF showed a dose dependent decrease (P≤0.05) of AST, ALT, LDH and CK-MB.
Table 1: Levels of serum AST, ALT, LDH, CK-MB, Total cholesterol (TC) and triglycerides (TG) in control and experimental rats treated with PEF
Table 2: Levels of serum total cholesterol (TC) and triglycerides (TG) in control and experimental rats treated with PEF
Values are mean ± S.D., n = 6.Statistical significance: ap≤0.05 – ISO group differs significantly from normal control group.bp≤0.05 –PEF (10mg/kg) + ISO (6mg/100g), 20mg/kg PEF+ISO (6mg.100g) and 30mg/kg PEF+ISO (6mg/100g) groups differs significantly from ISO control group. Table 3 shows activities of GSH, GPx, CAT and levels of MDA in heart tissue of ISO control and PEF treated experimental groups. PEF pre-treated groups showed a significant (P≤0.05) increase in the activities of GSH, GPx and CAT when compared to ISO control groups. MDA level was decreased significantly (P≤0.05) in PEF pre-treated groups than the ISO control groups.
Table 3: Activities of GSH, GPx, CAT and levels of MDA in heart tissue of control and experimental rats treated with PEF
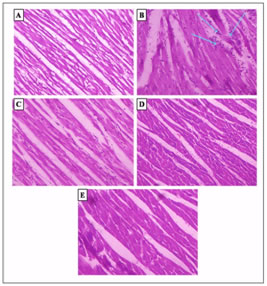
Values are mean ± S.D., n = 6.Statistical significance: ap≤ 0.05 – ISO group differs significantly from normal control group. bp≤0.05 –PEF (10mg/kg) + ISO (6mg/100g), 20mg/kg PEF+ISO (6mg.100g) and 30mg/kg PEF+ISO (6mg/100g) groups differs significantly from ISO control group. Effect of PEF treatment on histopathological changes of rat myocardium. The microscopic observations of myocardial histoarchitecture were qualitatively graded on the basis of myonecrosis, inflammatory cells and edema. The myocardium of control group showed a normal histoarchitecture (Fig.1A). Myocardium of ISO control rats showed massive necrosis of myofibers with cell infiltration, edema and increased connective tissue among myocardial fibers along with extra vasation of red blood cells (Fig. 1B). Pre-treatment with PEF at a dose of 10mg/kg and 30mg/kg in ISO- treated rats showed mild edema and inflammatory cells as compared to ISO control group (Fig.1 C and E). PEF pre-treated at a dose of 20mg/kg. b.w. showed almost the absence of myonecrosis, edema and inflammation (Fig.1D).
A) Normal control; (B) ISO control, (6 mg/100g s.c.); (C) PEF (10 mg/kg) + ISO; (D) PEF (20 mg/kg) + ISO; (E) PEF(30 mg/kg) + ISO.
DISCUSSION Petroleum ether fraction showed maximum efficacy in combating ISO induced myocardial infarction. This is manifested by improved cardiac function, reduced oxidative stress and improved antioxidant defence. We also found that PEF reduced myocardial necrosis and preserved myofibrillar structure histologically. Isoproterenol produces relative ischemia or hypoxia due to myocardial hyperactivity and coronary hypotension and induce myocardial ischemia due to cytosolic Ca2+overload 4-5. The oxidative stress may be exerted through quinone metabolites of isoproterenol, which reacts with oxygen to produce ROS 6. When myocardial cells, containing AST, ALT, CPK, and LDH, are damaged or destroyed due to deficient oxygen supply or glucose, the cell membrane becomes permeable or may rupture, which results in the leakage of enzymes. This might be due to the damage caused to the sarcolemma by the β-agonist that has rendered it leaky 7. This accounts for the increased activities of these enzymes in the serum of rats with myocardial ischemia-induced by isoproterenol which is in line with previous reports 8-10. The PEF pre-treatment (10, 20 and 30 mg/kg) blocked the increase of marker enzymes significantly in a dose dependent manner, indicating the cytoprotective activity of PEF. Lipid metabolism plays an important role in myocardial necrosis produced by ischemia. The significant increase in serum total cholesterol (TC) and triglycerides were observed in ISO control rats. PEF pre-treatment significantly decreases the levels of TC and TG in serum. This indicates the hypolipidemic activity of the extract. Increased levels of MDA in animals treated with ISO reflect excessive formation of free radicals by auto-oxidation of ISO and greater formation of lipid peroxides, resulting in severe damage to the myocardium 11. The ISO-elevated MDA levels were significantly decreased by the PEF pre-treatment, probably by preventing formation of lipid peroxides from fatty acids of the myocardium. Reduced glutathione is one of the most abundant non-enzymatic antioxidant bio-molecules present in tissues 12. It is a major non-protein thiol in living organism which plays a central role in coordinating the body’s antioxidant defence process. Its functions are removal of reactive oxygen species, such as H2O2, superoxide anions, and alkoxy radicals; maintenance of membrane protein thiols; and provision of a substrate for GPx and glutathione -S- transferase (GST) 13. Decreased GSH levels in ISO treated rats may be due to its increased utilization to augment the activities of GPx and GST. GSH is known to participate in the detoxification of electrophilic xenobiotics. Perturbation of GSH status of a biological system can lead to serious consequences. The decreased activities of glutathione peroxidase in the heart of ISO induced myocardial infarction might be due to decreased availability of their substrate, reduced glutathione. Inactivation of the enzyme GPx in the heart tissue leads to the accumulation of oxidized glutathione which in turn inactivates enzymes containing SH groups 14. The GSH and GPx levels depleted by ISO were significantly restored by PEF pre-treatment. Histopathological examination of heart tissue of group 2 rats showed myocardial necrosis and separation of myocardial fibres with inflammatory mononuclear infiltrate whereas the examination of heart tissue of PEF pre-treated groups (10mg, 20mg and 30mg/kg) showed protective effect in a dose dependent manner by reduced histological changes as compared to ISO myocardial infracted rats. The effective dose of PEF was 20mg/kg, which showed maximum cardioprotective effect by increasing the antioxidant status, decreasing the serum cardiac marker enzymes, serum cholesterol and triglyceride, lipid peroxidation and also by the absence of myonecrosis, edema and inflammation in histopathology.
CONCLUSION The result of serum biochemical parameters, level of cardiac lipid peroxides, tissue antioxidants and histopathological studies together support the highly potent cardioprotective and antioxidant activity of PEF. The observed antioxidant and cardioprotective activity of PEF in ISO induced MI may be due to the presence of the identified phytochemical constituents such as oleanolic aldehyde and vernolic acid. However, further studies are needed to comprehend the different mechanisms by which the petroleum ether sub fraction of methanolic extract of G. gummifera root exert its cardioprotective effect on ISO induced MI in rats.
REFERENCES
|
 Home
Home