Evaluation of bilirubin interference on amp buffer-based analysis of alkaline phosphatase activity in human serum
Kalpeshkumar Chhaganbhai Nakarani1, Zeel S Patel2, Vilas U Chavan3*
1Tutor, 2M.Sc. (MLT) Student, 3Professor & HOD, Department of Biochemistry , Surat Municipal Institute of Medical Education and Research, Surat, Gujarat, INDIA
Email: dr.kalpesh.c.nakrani@gmail.com
Abstract Background: Clinical biochemistry laboratory uses various methods for measuring the activity of alkaline phosphatase in serum. Many techniques are subject to interference from variety of sources. The aim of present study is to observe the effect of bilirubin interference on measured activity of alkaline phosphatase. Method: Present study of bilirubin interference effect was carried out as per the Clinical and Laboratory Standard Institute (CLSI) document EP07-A2. Result: There is a significant interference of bilirubin in analysis of activity of alkaline phosphatase. The experiment designed with five different concentration of bilirubin showed that bilirubin exerts negative interference in linear pattern with the estimation of alkaline phosphatase activity. Conclusion: Bilirubin has negative interference on analysis of alkaline phosphatase activity. In patients having hyperbilirubinemia, alkaline phosphatase alone should not be used as diagnostic marker.
Key Words: Alkaline phosphatase, Bilirubin, Interference.
INTRODUCTION
In clinical chemistry, interference can be defined as a cause of clinically significant bias in the measured analyte concentration due to the effect of another component or property of the sample. Interfering substances can be a significant source of error in clinical biochemistry laboratory measurements. Any measurement procedure, whether qualitative or quantitative, may be subject to interference. Potential interfering substances can be metabolites, drugs, sample preservatives, contaminants or sample matrix itself 1. Three principal contributors of inaccuracy (total analytical error) are imprecision, method-specific bias and sample-specific bias.2,3 Frequently imprecision and method-specific bias are estimated in measurement procedure evaluation but sample-specific bias (interference) is wrongly considered as a problem with specific sample.4,5 For an individual patient, such sample-specific bias is dependent on concentration of interfering substance in that particular sample and because of such sample to sample variation in bias could lead to erroneously interpretation which can worsen the clinical outcome. Serum alkaline phosphatase is one of the important clinical biochemistry laboratory tests used to screen for hepatobiliary diseases and bone diseases associated with increase osteoblastic activity 6. Higher serum alkaline phosphatase levels have been reported to be associated with incident as well as prevalence of metabolic syndrome which leads to increase mortality 7, 8, 9. Bilirubin, a catabolic end product of heme is found to interfere in the estimation of various biochemical parameters like creatinine, glucose, alkaline phosphatase, cholesterol, triacylglycerol, uric acid, and some enzymes 10, 11, 12. Bilirubin is present in the blood in several distinct forms as conjugated and unconjugated bilirubin. Additionally, bilirubin photoisomers may be found in blood of the neonates 13. In patients with liver diseases, along with alkaline phosphatase, bilirubin level is also elevated. Bilirubin can interfere through two mechanisms: spectrophotometric interference due to its ability to absorb light between 400 to 540 nm and chemical interference as it consumes hydrogen peroxide which is intermediate in many reactions e.g. cholesterol, glucose, uric acid, triacylglycerol 14. Based on present knowledge, our study is primarily aimed to find out whether high concentration of bilirubin interferes with the detection of alkaline phosphatase in our laboratory settings. Secondly if bilirubin interference found significant then we also intend to evaluate effect of different concentrations of bilirubin on estimation of activity of alkaline phosphatase.
MATERIALS AND METHODS
This study was carried out in the Department of Biochemistry at Surat Municipal Institute of Medical Education and Research (SMIMER), Surat, Gujarat, India, during April-2019 to June-2019. This project was approved by institutional ethical committee of SMIMER. This study was experimental work carried out as per Clinical and Laboratory Standard Institute (CLSI) approved guideline EP7-A2 15.
Instruments and Reagents:
Instruments:
Fully automated clinical chemistry analyzer Erba-XL-640 (Transasia Bio-Medicals Ltd. Mumbai, India) was used for bilirubin and alkaline phosphatase estimation. Electronic balance ABJ 320-4 (Kern and Sohn, Germany) was used for weighing reagents. Both instruments were calibrated as per standard protocol.
Reagents:
Alkaline phosphatase activity was measured by AMP (2-amino-2-methyl-1-propanol) buffer and pNPP (para-nitrophenylphosphate) substrate-based kit (Randox Laboratories India Pvt Ltd, Bengaluru, India) [16]. Concentration of bilirubin was measured by kit based on dichloroaniline method (Meril Diagnostics, Vapi, India) [17]. Both kits were calibrated using Randox calibrator and quality check was made by Randox quality control sera. Commercially available analytical grade anhydrous bilirubin powder was used for making bilirubin stock solution.
Methods:
Preparation of bilirubin stock solution:
Accurately weighed 60 mg of anhydrous analytical grade bilirubin powder was dissolved in 5 ml of 0.4% NaOH. This solution was constantly swirled and step wise (100µl in each step) supplementation of 0.4% NaOH was carried out until all the bilirubin powder dissolved completely. Such bilirubin stock solution was freshly prepared as and when required so that problems related to photolysis and degradation of bilirubin can be minimized. High concentration of bilirubin in stock solution is required because when we spike the sample with this bilirubin stock solution, minimum possible volume of stock solution should be used so that sample matrix modification is kept minimum [15]. Every time bilirubin stock solution prepared, we estimated concentration of total bilirubin 20 times in stock solution, calculated its mean ± SD and compared it with the calculated concentration. If calculated and estimated concentrations are in agreement then only bilirubin stock solution considered fit for experiment.
Preparation of Base Pool samples:
As per CLSI EP07-A2 guideline, Analyte test concentration for interference testing should include medical decision levels as far as possible. In present study we prepared three separate pooled serum of low, medium and high Alkaline phosphatase activity. Fresh serum samples were obtained from apparently healthy individuals who had not taken any medications including vitamins, dietary supplements in past 1 month and had not undergone any surgical procedure in last 1 year. Samples from pregnant women were also excluded from the study. Samples should not have any signs of icterus, hemolysis and lipemia. Samples having Alkaline phosphatase activity lower than 40 U/L were used for preparing low base pool, between 40 to 120 U/L were used for preparing medium base pool and more than 120 U/L were used for preparing high base pool sera. Bilirubin is endogenously produced by breakdown of heme; therefore, it is necessary to keep bilirubin concentration minimum in the base pool sera. for purpose of keeping low bilirubin concentration in pooled sera, the samples which had bilirubin level more than 1mg/dL were not used for preparation of pooled sera. finally, the activity of alkaline phosphatase and concentration of bilirubin was measured 20 times and their mean and SD were estimated to establish base line characteristics of pooled sera.
Preparation of control and test pool sera:
As per CLSI guideline, the volume of bilirubin stock solution which is added to base pool serum should be lower than 1/20th fraction (5%) of base pool serum to minimize the alteration in sample matrix [15]. Control pool preparation should be exactly as test pool in all respect except the test interferent is replaced with the same volume of solvent used to prepare bilirubin stock solution. As bilirubin is also present endogenously in base pool, final concentration of bilirubin should be measured by laboratory. Control pool was prepared by adding 300µl of 0.4% NaOH in the 5700µl of base pool. Three different control pool were prepared in line with low, medium and high base pool. Test pools were prepared by adding 300µl of bilirubin stock solution in the 5700µl of base pool. So total 6 solutions (3 control pools and 3 test pools) were prepared as we have 3 different low, medium and high base pools.
Number of replicates:
The number of replicates needed to detect various interference effect with 95% confidence level (α=0.05) and 95% power (β=0.05) were calculated based on maximum allowable error derivative (dmax) and within-run repeatability (SD) of the analytic technique. In present study, for the purpose of calculating dmax, the maximum allowable error was determined based on the biological variation of alkaline phosphatase activity in serum. Ricos et al (1999) estimated total error (TE) of serum alkaline phosphatase based on biological variation as 11.7% [18] and we considered this value in present study. The dmax of each pool was calculated by below mentioned formula.
Within-run repeatability of alkaline phosphatase was determined by analyzing each base pool (low, medium and high) 20 times and their standard deviation was calculated. These calculated dmax and SD values were used in following formula to find number of required replicates (n) [19]. In case of non-integer value, rounding up to the next integer value was considered.
Where, z values were used from standard statistical table for percentile for confidence level and power.
Sample analysis:
The test (T) and control (C) samples were analyzed in alternate order e.g. C1T1C2T2C3T3….CnTn. To prevent the effect of carryover from test samples to control samples, additional control serum samples were intermittently added e.g. C1T1CxCxC2T2CxCxC3T3…CxCxCnTn, where Cx is the additional control serum sample. Later on, the results from additional control serum results were discarded. Analysis of both serum bilirubin and alkaline phosphatase were carried out simultaneously.
Data analysis:
Control and test pool analysis data were used to compute the ‘point estimate’ of the observed interference effect, dobs, as the difference between the means of the test and control samples.
The cut-off value for two-sided test, dc, was used to determine whether null hypothesis should be accepted or rejected. In present study, null hypothesis states that bilirubin do not interfere with the estimation of alkaline phosphatase activity (dnull = 0). The cut-off value, dc, can be determined by following formula
Where, dnull = Value stated in null hypothesis = 0 in present study
n = number of replicates
If the point estimate, dobs, is less than or equal to the cut-off value, dc, than null hypothesis should be accepted otherwise alternate hypothesis i.e. bilirubin interferes with the estimation of alkaline phosphatase activity, should be accepted. If the interferent effect is found, then we need to carry out dose response series of interferent to determine the degree of interference as a function of the interferent concentration.
Preparation of five level dose-response samples:
A series of test samples, systematically varying only in the concentration of interferent, is prepared by making proportionate mixture of two pools, one at highest concentration to be tested and the other at lowest. In this part of experiment, earlier used test pool sample was used as high bilirubin concentration sample and control pool sample was used as low bilirubin concentration sample. The proportionate mixture of high and low bilirubin concentration serum for all three alkaline phosphatase level pools are shown in table-1.
Table 1: Preparation method of dose-response samples along with measured concentration of bilirubin in each pool
Series No. |
Proportionate mixture |
Measured concentration of bilirubin |
Low bilirubin concentration sample |
High bilirubin
concentration sample |
Low alkaline phosphatase pool |
Medium alkaline
phosphatase pool |
High alkaline phosphatase pool |
1 (L) |
400 µl |
0 µl |
0.53 mg/dl |
0.6 mg/dl |
0.63 mg/dl |
2 (3L + 1H) |
300 µl |
100 µl |
18.7 mg/dl |
18.5 mg/dl |
17.6 mg/dl |
3 (2L + 2H) |
200 µl |
200 µl |
37.06 mg/dl |
36.35 mg/dl |
34.6 mg/dl |
4 (1L + 3H) |
100 µl |
300 µl |
55.3 mg/dl |
54.2 mg/dl |
51.6 mg/dl |
5 (H) |
0 µl |
400 µl |
73.6 mg/dl |
72.1 mg/dl |
68.7 mg/dl |
In present study a total five concentrations were analyzed in triplicates within the same analytical run. Further to minimize drift effects, all the samples and replicates were run in random order sequence which was generated from table of random numbers.
Data analysis of dose-response sample:
Observed interference effect at each bilirubin concentration sample was calculated by taking average alkaline phosphatase activity of low bilirubin concentration pool and deducting it from all the individual result of alkaline phosphatase activity. These results were plotted with observed effect on the y-axis and the interferent concentrations on x-axis to examine the shape of dose-response relationship. If data appeared randomly distributed about a straight line, linear least square regression was applied to determine slope, intercept and residual error from individual observations. If linear relationship found between interferent concentration and analyte, the regression slope represents the bias per unit of interferent and the y-intercept represents the correction factor for the endogenous interferent concentration.
OBSERVATIONS AND RESULTS
Table 2: Evaluation of the interfering effects of total bilirubin on the determination of alkaline phosphatase
Description of data |
Level of alkaline phosphatase in pool |
Low |
Medium |
High |
ALP activity in base pool (U/L) |
40.8 |
88.1 |
184 |
Total allowable error (TE%) |
11.7% |
11.7% |
11.7% |
dmax |
4.7 |
10.3 |
21.5 |
SD |
0.63 |
0.31 |
1.05 |
No of required replicates |
3 |
3 |
3 |
ALP activity in control pool (U/L) |
38.6 |
81.6 |
172.3 |
ALP activity in test pool (U/L) |
28 |
72.3 |
167.3 |
Error caused by interference |
-27.46% |
-11.4% |
-2.9% |
dobs |
-10.6 |
-9.3 |
-5 |
dc |
0.7 |
0.35 |
1.18 |
Interference judgement |
Yes |
Yes |
Yes |
Evaluation of interferent effect of total bilirubin was illustrated in Table-2. As we can see from table-2, three pools of alkaline phosphatase were prepared. Alkaline phosphatase activity was 40.8 ± 0.63 U/L, 88.1 ± 0.31 U/L and 184 ± 1.05 U/L in low pool, medium pool and high pool respectively. Based on calculated dmax values, no of required replicates were 3 for all three pools. After preparing control and test pools their alkaline phosphatase activity was measured 3 times. Mean alkaline phosphatase activity in control pool was 38.6 U/L, 81.6 U/L and 172.3 U/L in low, medium and high pool respectively whereas activity in test pool was 28 U/L, 72.3 U/L and 167.3 U/L in low, medium and high pool respectively. The observed difference was more than the cutoff limit in all three pools which indicated that interference exists for all three alkaline phosphatase pools.
Dose response experiment of bilirubin interference on alkaline phosphatase analysis:
Different concentrations of bilirubin interference error can be estimated by linear least square regression analysis as shown in figure 1-3.


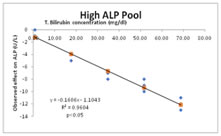
Figure 1 Figure 2 Figure 3
Figure 1: Dose response experiment of bilirubin interference (Low ALP); Figure 2: Dose response experiment of bilirubin interference (Medium ALP); Figure 3: Dose response experiment of bilirubin interference (High ALP)
As shown in figure 1, 2 and 3, as total bilirubin concentration increases in serum, corresponding decrease in alkaline phosphatase activity occurs at all three levels of alkaline phosphatase. It also shows that bilirubin interference is maximum when alkaline phosphatase activity is low and bilirubin concentration is higher.
DISCUSSION
In clinical chemistry, both pre- and post-analytical factors can cause erroneous results. Identification and evaluation of interferences is recommended as an integral part of the Quality Control Program thereby assuring patient safety and high-quality healthcare 20. The interference caused by bilirubin is one of the main concerns for the laboratory medicine and could result in positive or negative sample-specific bias which causes increase in the total analytical error and leads to erroneous reporting of patient results. Total analytical error consists of three principle components: imprecision, method-specific bias and sample specific bias 2,3. Evaluation of measurement procedures more frequently estimates only imprecision and method-specific bias. Sample specific bias (i.e. interference) is often viewed as an isolated problem with specific samples, rather than as a measurable characteristic of the procedure. Interference study might be best carried out by reagent and instrument manufacturers rather than clinical laboratories, however clinical laboratories have to ensure that measurement procedures are able to meet the needs of their clients so laboratories should also investigate discordant results to identify any interfering substances and objective feedback should be provided to manufacturer for continuous improvement in quality 15. This study was carried out in two parts: in part one, ‘interference screen’ was carried out in which ‘paired difference testing’ between bilirubin spiked sample as test and same sample pool without added bilirubin as control pool were evaluated. Relatively higher bilirubin was added in test pool to simulate ‘worst case’. If part one shown clinically significant bias then only part two of experiment was carried out. In part two, analysis of dose response series was carried out to determine the degree of interference as a function of interferent concentration. As shown in table-2, high concentrations of bilirubin have negative interference on analysis of alkaline phosphatase activity. In low, medium and high alkaline phosphatase pool bilirubin causes -27.46%, -11.4% and -2.9% measurement of alkaline phosphatase activity. Similar negative interference of bilirubin was also observed in several other studies 21, 22. Figure 1-3 shows effect of incrementing bilirubin concentration on measurement of alkaline phosphatase activity. The regression linear formula can also be used by clinical laboratories to predict the actual activity of alkaline phosphatase in clinical samples when bilirubin concentration is higher. As shown in figure 1-3, we observed maximum bilirubin interference whenever there is low alkaline phosphatase or higher bilirubin level, better predictor of bilirubin interference can be ratio of bilirubin to alkaline phosphatase rather than just bilirubin level however further studies are required to reach at that conclusion. In general, mechanism of interference can be because of chemical artifacts, detection artifacts, physical artifacts, enzyme inhibition, non-specificity, cross-reactivity or water displacement 23, 24 however the actual mechanism involved in negative bilirubin interference on analysis of alkaline phosphatase is not clear but bilirubin by being reducing agent can weaken electron and proton transmission which can lead to reduced enzyme activity 25. Frequently in patients with liver diseases, simultaneous increase in bilirubin and alkaline phosphatase levels are observed, our results suggest that in icteric samples, alkaline phosphatase alone should not be used as diagnostic or prognostic marker as it may produce clinically unreliable results. In case of icteric sample, total bilirubin concentration should be estimated and regression formula shown in figure 1-3 should be applied to predict the magnitude of interference on alkaline phosphatase activity. However, it is advisable for laboratories to carry out interference experiment in their own clinical settings. Our study had certain limitations: first, we used commercially available bilirubin to spike the samples, its properties might be different from in vivo bilirubin. Second, the test samples were diluted and so matrix might be modified so it may not represent the ideal sample of clinical settings.
CONCLUSIONS
In present study, interference effect of bilirubin on estimation of alkaline phosphatase was carried out as per CLSI guideline EP07-A2 at clinical biochemistry laboratory of tertiary care hospital. Important findings of this study are:
- Bilirubin has negative interference on analysis of alkaline phosphatase activity.
- In patients having hyperbilirubinemia, alkaline phosphatase alone should not be used as diagnostic marker.
- In case of hyperbilirubinemia, one of regression formula derived in this study can be used to predict the magnitude of interference on alkaline phosphatase activity.
REFERENCES
- Kaplan LA, Pesce AJ. Interferences in chemical analysis In: Kaplan LA, Pesce AJ, eds. Clinical Chemistry-Theory, analysis and correlation. 5th edn. St. Louis, USA: The C.V. Mosby Co.; 2009: p.310-313
- Kringle RO, Bogavich M. Statistical procedures. In: Burtis CA, Ashwood ER, eds. Tietz Textbook of Clinical Chemistry. 3rd ed. Philadelphia: W.B. Saunders Co.; 1999:265-309.
- Krouwer JS. Estimating total analytical error and its sources: Techniques to improve method evaluation. Arch Pathol Lab Med. 1992;116:726-731.
- Lawton WH, Sylvestre EA, Young-Ferraro BJ. Statistical comparison of multiple analytic procedures: Application to clinical chemistry. Technometrics. 1979;21:397-409.
- Krouwer JS. How to improve total error modeling by accounting for error sources beyond imprecision and bias. Clin Chem. 2001;47(7):1329-1331.
- M Panteghini, R Bais. Serum Enzymes. In: N Rifai, A Horwath, C Wittwer, editors. Tietz textbook of Clinical Chemistry and Molecular Diagnostics, 1st SEA Edn, India: RLX (an imprint of Elsevier); 2018. Pp. 415-416
- Hanley AJ, Williams K, Festa A, Wagenknecht LE, D’Agostino RB Jr, Haffner SM. Liver markers and development of the metabolic syndrome: the insulin resistance atherosclerosis study. Diabetes. 2005; 54(11):3140–3147.
- Zelle DM, Corpeleijn E, van Ree RM, et al. Markers of the hepatic component of the metabolic syndrome as predictors of mortality in renal transplant recipients. Am J Transplant. 2010; 10(1):106–114.
- Krishnamurthy VR, Baird BC, Wei G, Greene T, Raphael K, Beddhu S. Associations of Serum Alkaline Phosphatase with Metabolic Syndrome and Mortality, Am J Med 2011; 124: 566.e1-566.e7
- Agarwal S, Vargas G, Nordstrom C, et al. Effect of interference from hemolysis, icterus and lipemia on routine pediatric clinical chemistry assays. Clin Chem Acta 2015; 438:241-245
- Steen G, Vermeer HJ, Naus AJ, et al. Multicenter evaluation of interference of hemoglobin, bilirubin and lipids on Synchron LX-20 assays. Clin Chem Lab Med 2006; 44: 413-419
- Ji JZ, Meng QH. Evaluation of the interference of hemoglobin, bilirubin, and lipids on Roche Cobas 6000 assays. Clin Chem Acta 2011; 412: 1550-1553
- Yamauchi Y, Yamanouchi I. Initial response of serum bilirubin levels to phototherapy. Biol Neonate 1991; 60: 314-319
- AM Simundic, N Nikolac, WG Guder. Preanalytical Variation and Preexamination Processes. In: N Rifai, A Horwath, C Wittwer, editors. Tietz textbook of Clinical Chemistry and Molecular Diagnostics, 1st SEA Edn, India: RLX (an imprint of Elsevier); 2018. Pp. 92-93
- Clinical and Laboratory Standards Institute (CLSI). Interference Testing in Clinical Chemistry; Approved Guideline—Second Edition. CLSI document EP7-A2 (ISBN 1-56238-584-4). Clinical and Laboratory Standards Institute, 940 West Valley Road, Suite 1400, Wayne, Pennsylvania
- Randox ALP kit. For photometric measurement of alkaline phosphatase activity in serum based on IFCC recommended method using AMP buffer. Kit insert. Randox Laboratories Pvt Ltd, Bengaluru, India (www.randox.com)
- CliniQuant Bilirubin kit. For photometric estimation of total and direct bilirubin concentration based on DCA method. Kit Insert. Meril Diagnostics Pvt Ltd, Vapi, Gujarat, India (www.merillife.com)
- Ricos C, Alvarez V, Cava F, Garcia-Lario JV, Hernandez A, Jimenez CV, et al. Current databases on biological variation: pros, cons and progress. Scand J Clin Lab Invest. 1999;59(7):491–500.
- Desu MM, Raghavarao D. Sample Size Methodology. San Diego: Academic Press, Inc.; 1990:7,9,30.
- Saibaba KS, Bhaskar MV, Rao PV, Ramana GV, Dakshinamurty KV. Interferences in clinical chemistry analysis. Indian J Clin Biochem 1998;13:55–62.
- Zhong Wang, Hongyan Guo, Ying Wang, et al. Interfering effect of bilirubin on the determination of alkaline phosphatase, Int J Clin Exp Med 2014; 7(11): 4244-4248
- Noha A. Darag, Nassr E. Mohamed. Interfering effect of bilirubin on the measurement of alkaline phosphatase activity. Euro j bio pharma sci 2018; 5(10): 29-32.
- Apple FS, Koch DD, Graves S, Ladenson JH. Relationship between direct-potentiometric and flame-photometric measurement of sodium in blood. Clin Chem. 1982;28:1931-1935.
- Durst RA, Siggard-Andersen O. Electrochemistry. In: Burtis CA, Ashwood ER, eds. Tietz Textbook of Clinical Chemistry. 3rd ed. Philadelphia: W.B. Saunders Co.; 1999.
- Pyles LA, Stejskal EJ, Einzig S. Spectrophotometric measurement of plasma 2-thiobarbituric acid-reactive substances in the presence of hemoglobin and bilirubin interference. Proc Soc Exp Biol Med 1993; 202: 407-419.