|
Table of Content - Volume 18 Issue 1 - April 2021
Genetic mutations in poorly differentiated thyroid cancer and follicular differentiated thyroid cancer
Ramesh Bangaraiahgari1, Rajesh Bangaraiahgari2, Rafi Md3, Rajkiran reddy B4, Ramakanth Bhargav Panchangam5*
{1Associate Professor, 3Professor, Department of Biochemistry} {2Associate Professor, Department of Anatomy} Surabhi Medical College, Hyderabad, Telangana, INDIA. 4Senior Research Fellow, Department of Genetics and Laboratory, Sunrise Hospitals, Hyderabad, INDIA. 5Senior Consultant Endocrine Surgeon, Department of Endocrine and Metabolic Surgery, Endocare Hospital, Vijayawada, AP, INDIA. Email: endoanswers@gmail.com
Abstract Background: Poorly differentiated thyroid cancer (PDC) is a rare form of differentiated thyroid cancer with a prognosis in between well differentiated (DTC) and anaplastic cancers (ATC). Genomics is one of the definitive modalities to study PDC. ATC tends to express P53 and KMT2D gene mutations more frequently than DTC. DTC tends to express BRAF and RAS gene mutations more frequently than ATC. In this context, we set out to study the prevalence and impact of the somatic mutations in PDC, DTC and ATC. Material and methods: This retrospective study was conducted on surgically managed thyroid cancer patients. Diagnosis was based on biochemical confirmation, imaging, fine needle aspiration cytology and later confirmed by histopathology. We selected 10 PDC, 4 ATC and 32 DTC cases. Tumour tissue samples collected from operation theatre were subjected to to DNA extraction, cDNA preparation, amplification and application of six of primers was performed for mutational analysis of BRAF, RAS (N,K,H), P53 and KMT2D genes. Results: Heterozygous mutations in KMT2D gene and missense mutation in P53 gene were found in 6/10 (60 %) and 4/10 (40%) of PDC cases respectively. In ATC, KMT2D mutations were seen in 2/4 (50%) and 4/4 (100%) cases. In WDTC, mutations were found in neither of these two genes. Homozygous mutations in N-RAS were found in 19/32 (59.3 %) of DTC, 3/10 (30 %) of PDC and 1/4 (25%) of ATC cases, respectively. Homozygous mutations were found in BRAF gene in 23/32 (72 %) of DTC, 4/10 (40%) of PDC and 2/4 (50%) of ATC cases, respectively. Conclusions: Our study shows a distinct pattern of mutations in BRAF, RAS, KMT2D and P53 genes suggesting correlation between the gene function and cancer cell differentiation in thyroid cancer. Key words: Poorly differentiated thyroid cancer; thyroglobulin; thyroidectomy; total thryroidectomy; Anaplastic cancer.
INTRODUCTION Ever since, the first report of poorly differentiated thyroid cancer (PDC) by Sakamoto, 1 it has been evolving as a distinct clinicopathological entity. There are conflicting reports about the status of PDC as a distinct entity in literature. Debate over its pathological definition and management continues due to its rarity and lack of prospective clinical trials or studies. 2-4 Further, the widely held hypothesis justified by available evidence, is that the prognosis of PDC is intermediate between follicular differentiated thyroid cancer (DTC) and anaplastic or undifferentiated thyroid cancer.5-7 But, this finding is a debatable issue in literature. Genomics is one of the definitive modalities to resolve this conundrum. ATC and PDC tends to express P53 and KMT2D gene mutations more frequently than WDTC. 8 Though, P53 gene mutations are found rarely in WDTC, these mutations are primarily correlated with poor differentiation and prognosis of cancers. 8 WDTC tends to express BRAF and RAS gene mutations more frequently than ATC. 8,9 These mutations portend a more indolent course and better prognosis in thyroid cancer. Studies on the prevalence and functional significance of these gene mutations in PDC are scanty. In this context, we set out to study the prevalence of these somatic mutations and their clinical relevance in PDC in comparision with WDTC and ATC.
MATERIAL AND METHODS This is a retrospective spanning a period from 2009 to 2015 with a minimum of five year followup. Out of 2464 cases of thyroid cancer treated in our Endocrine surgery department, we selected 10 PDC, 4 ATC and 32 WDTC cases to be included in this study. All clinical, investigative, pathological, treatment and follow-up details were retrieved and anlaysed systematically. Tumour tissue samples were taken from ex-vivo thyroidectomy/ debulking/ trucut specimen within operation theatre. Inclusion criteria was all thyroid cancer cases with histopathology of partial or florid poorly differentiated thyroid cancer (insular, trabecular, solid variants); well differentiated follicular cell derived thyroid cancer variants – classical (Not otherwise specified – NOS) subtype of papillary thyroid cancer (PTC), follicular thyroid cancer (FTC); anaplastic undifferentiated cancer, either operated or diagnosed on trucut biospsy (in inoperable cases), with a minimum followup period of 5 years.. Exclusion criteria are medullary thyroid cancer, Hurthle cell cancer, other biologically aggressive variants of PTC such as tall cell, columnar cell, oncocytic, diffuse sclerosing variants; cases operated elsewhere; those with incomplete records and lost to follow-up cases. This study complied with the international ethical norms of the Helsinki Declaration — Ethical Principles for Medical Research Involving Human Subjects, 2004. 10 Institutional ethical committee approval was obtained. Informed consent was obtained from all the included members of the cohort. Definitions and standards employed for this study
Genetic methodology After appropriate processing of thyroid tissue samples, DNA extraction and cDNA preparation was done. DNA quality was checked by agarose gel electrophoresis for any degradation or RNA contamination. Two types of mutations were looked for – known (mutations already reported in the database of SNPs) and unknown (mutations never reported before). Known mutations and single nucleotide polymorphisms (SNPs) were analyzed with restriction fragment length polymorphism analysis. Transcriptomic sequencing of exonic segments of above genes, are analysed. For unknown or novel mutations, we planned to select hotspots on sequencing and study them. The structural and functional analysis of the gene segments consisting mutations, was performed. Statistical analysis Statistical analysis was performed using IBM SPSS software. Descriptive statistics were analysed with t-test and Chi-square tests. Recurrence free survival and overall survival rates as a outcome of PDC versus PDA were estimated by Kaplan-Meier product limit estimate method, comparisions made by Log rank test. Univariate and multivariate analysis were done using general linear model. P value of < 0.05 was taken as statistically significant probability cutoff.
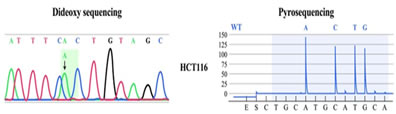
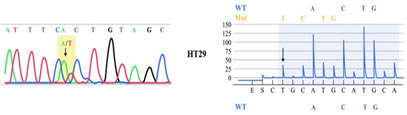
RESULTS Mean follow up in DTC, PDC and ATC were 64 ± 23.5 months (60 – 123), 53 ± 20.5 months (42 – 94) and 3.5 SD 2.3 (2-13 months) respectively. Mean age and gender ratio were 41 ± 12 years (16 – 76); M:F = 1:1.3, 54 ± 10.5 years (36 – 81); 1:1.6 and 62 SD 3 years (60-74); 1:1 in DTC, PDC and ATC respectively. Total thyroidectomy was possible in 96%, 80% and 0% of DTC, PDC and ATC respectively. No cases of permanent hypoparathyroidism or recurrent laryngeal nerve palsy were noted in postoperative period. Frequency distribution and statistical significance of various clinical and survival parameter are detailed in Table 1. The number of subjects according to TNM group staging were 39%, 19%, 23% and 18% in DTC and 10%, 19%, 23% and 48% in PDC respectively. According to AMES risk stratification, 1.7:1 and 2.5:1 ratio of high risk versus low risk in DTC and PDC respectively. Histopathology was defined by presence of any STI component seen focally in 32 cases of PDA and predominantly (> 10% area) in 29 cases of PDC. Radio-iodine ablation was utilized in 45% (DTC); 29% (PDC) and in 0% of ATC. Adjuvant EBRT was used in one case of PDC and two cases of ATC (palliative EBRT). One PDC and two ATC cases received chemotherapy. The extrathyroidal invasion, lymphadenopathy rate, mean tumour size, metastasis rate, recurrence rate and survival rates were more in ATC> PDC versus DTC, reaching statistical significance, suggestive of a aggressive tumour biology in ATC>PDC vis-a-vis DTC. Nodal recurrence (PDC = 29%; DTC = 3 %) and systemic metastasis was 41% in PDC (synchronous = 24%; metachronous = 17%); 3% in DTC (synchronous = 3%; metachronous = 0%). Five year event free survival (EFS) and overall survival (OS) was 950% and 100% in DTC, 42% and 44% in PDC, 0% and 0% in ATC, respectively. Among AMES and TNM variables, only metastases affected EFS (P value = < 0.05) and none affected OS. As shown in Table 2 - age of the patient, presence of distant metastasis and tumour size, but not ETI had statistically significant effect on overall survival and recurrence free survival. Homozygous mutations were found in BRAF gene in 23/32 (72 %) of DTC, 4/10 (40%) of PDC and 2/4 (50%) of ATC cases, respectively as illustrated in Fig 2. Homozygous mutations in N-RAS were found in 19/32 (59.3 %) of DTC, 3/10 (30 %) of PDC and 1/4 (25%) of ATC cases. The differences were statistically significant. No H-RAS or K-RAS mutations were found in any of our cases. No novel mutations were found in our study. Heterozygous mutations in KMT2D gene and missense mutation in P53 gene were found in 7/10 (70 %) and 5/10 (50%) of PDC cases, respectively. In ATC, KMT2D gene and P53 gene mutations were seen in 2/4 (50%) and 4/4 (100%) cases, respectively. Representative mutations are illustrated in Fig 1 and 2. In WDTC, mutations were found in neither of these two genes.
Figure 1: Representative KMT2D gene mutation found in our study
Figure 2: Representative P53 gene mutation found in our study
Table 1: Comparative significance of clinicopathological and survival variables
*NA = Not Applicable Table 2: Univariate and multivariate analysis of prognostic factors
DISCUSSION Ever since Sakamoto proposed the term poorly differentitated carcinoma for this entity in 1983, 1 there has been intense effort to arrive at consensus on definition, categorization, prognostication and treatment strategies between institutions from diverse geographies. Sakamoto used the definition of presence of solid, trabecular or scirrhous patterns in follicular origin thyroid cancer as PDC with prognosis intermediate between DTC and ATC. This seminal article was followed by other reports with various inclusion criteria and prognostic outcomes. 5-7, 14-16 In 2004, World Health Organisation (WHO), came up with a more objective definition of PDC - “follicular neoplasms that show limited evidence of structural follicular cell differentiation and occupy both morphologically and behaviorally an intermediate position between differentiated (follicular and papillary carcinomas) and undifferentiated (anaplastic) carcinomas.” 13 To further streamline this entity, a consensus meet was held at Turin in 2006, which gave a multi-tiered diagnostic algorithm for PDC – “any or all of STI features, absence of typical nuclear features of PTC, mitoses (≥3 mitoses per 10 hpf), convoluted nuclei, or necrosis”. This stringent Turin criteria proved to be most widely accepted definition, inspite of room for errors in case of encapsulated lesions with high grade features and extent of PD areas. 17 Available literature and global experience placed PDC as an intermediate prognostic entity between DTC and ATC. 2-7 At one end of spectrum, DTC is a relatively indolent disease and ATC is a rapidly progressive and oftenly fatal disease at other end of spectrum in terms of prognosis. This tumour biology holds true irrespective of extent and modality of treatment instituted. But, reported prognosis varies within a grey zone between that of DTC and ATC. Evolution of genetic studies and ready availability of targeted genetic analysis opened up a new avenue to plug the lacunae in cancer tumour biology. Genomics is one of the definitive modalities to resolve this conundrum. In our study, we specifically tried to address the prognostic differences between PDC, DTC and ATC, through a genomic viewpoint in correlation with our clinic-pathological and followup data. We opine that PDC is a distinct clinicopathological entity between DTC and ATC. Moreover, as shown in our data, PDC appears to have an intermediate prognosis between DTC and ATC, suggesting a progressive tumour biology. Further, the significant difference in tumour size, ETI, metastases rate, survival rates between DTC, PDC and ATC shows that they are biologically different. Strengthening this speculation is also the availability of genetic studies suggestive of transition from DTC to PDC. They show increasing mutation rates of RAS, BRAF, RET-PTC, PPAR/ PAX8, WNT/B catenin genes. 17-20 Inactivating P53 mutations were significantly found in ATC and rarely in PDC. But they were not found in DTC. Our study shows a similar trend of increasing frequency of P53 mutations from DTC<PDC<ATC subtypes. Moreover, similar trend was found with KMT2D gene mutations. But, mutations were not found in either of KMT2D and P53 genes in DTC. On the contrary, BRAF and N-RAS mutations were found in decreasing order of frequency in DTC>PDC>ATC subtypes. A next generation sequencing study, showed that greater mutation burden was found in ATC compared to PDC. 8 Further that study showed that higher mutation frequency was associated with poorer prognosis, elderly age, higher grade and higher recurrence rates. 8 We hypothesize, that each particular gene malfunction, its expression have proportionate impact of grade, subtype and prognostic outcomes in PDC. As shown in our results, BRAF, N-RAS portended significantly better prognosis, while P53 and KMT2D gene mutations dictated poorer prognosis in thyroid cancer. Though some studies on PDC, showed better prognosis with BRAF and worser prognosis with RAS gene mutations, we found no such phenomenon. Thyroid differentiation score studies showed thatATC had profoundly lower mRNA levels of genes involved in iodine metabolism, justifying our hypothesis of progressive tumour biology. 8,9 Moreover, PDC and ATC are thought to arise from preexisting PTCs based on their frequent co-occurrence in the same tumor specimen, where they consistently share a driver mutations in BRAF and RAS genes. 21,22 Our study shows similar trend of co-occurrence of ATC, PDC with DTC in 50%, 35% of cases respectively, with shared driver mutations in BRAF, RAS genes.
CONCLUSIONS Our study shows a distinct pattern of mutations in BRAF, RAS, KMT2D and P53 genes suggesting correlation between the gene function and cancer cell differentiation in thyroid cancer.
REFERENCES
Policy for Articles with Open Access: Authors who publish with MedPulse International Journal of Pediatrics (Print ISSN: 2579-0897) (Online ISSN: 2636-4662) agree to the following terms: Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution License that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal. Authors are permitted and encouraged to post links to their work online (e.g., in institutional repositories or on their website) prior to and during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work.
|
|
 Home
Home