|
Table of Content - Volume 19 Issue 2 - August 2021
Influence of endothelial nitric oxide synthase gene intron 4 VNTR polymorphism on serum nitric oxide level among healthy south-Indian population
G Sasirekha1, A Veena Juliette2, S Siva3*
1Associate Professor, Department of Biochemistry, Madurai Medical College, Madurai, INDIA. 2Associate Professor, Department of Biochemistry, Kilpauk Medical College, Chennai, INDIA. 3Associate Professor, Department of Biochemistry, KAPV Government Medical College, Trichy, INDIA. Email: anusasi1984@gmail.com
Abstract Background: Nitric oxide (endothelium derived relaxing factor) is synthesized from L-arginine by nitric oxide synthases. Endothelial NOS is responsible for most of the nitric oxide produced under physiological conditions. Acting via cGMP NO cause smooth muscle relaxation, prevents platelet aggregation and acts as an anti-inflammatory agent thereby playing a vital role in regulating vascular tone. Various studies have postulated the role of Nitricoxide in vascular disorders like hypertension and atherosclerosis. This study is aimed to find out the influence of endothelial nitric oxide synthase gene intron 4 VNTR polymorphism on Serum nitric oxide level among healthy South-Indian Population. Genotype analysis was done on 282 randomly selected healthy individuals by polymerase chain reaction. Phenotype analysis of eNOS activity was done by measuring serum NOx level. Significantly lower eNOS activity (10.5 versus 18.3, P value is < 0.00001) among ‘a’ alleles when compared to ‘b’ alleles. We have found the presence of eNOS ‘a’ allele is found to decrease serum NOx level. As the distribution of ‘a’allele is lower among the population, a larger study is needed to find the role of eNOS intron4 gene in maintaining serum Nitric oxide level. Key Words: Nitric Oxide, Genotype, VNTR polymorphism, polymerase chain reaction
INTRODUCTION Nitric oxide (NO) is a potent bioactive gas with vasodilatory, anti-inflammatory, antithrombotic, antiproliferative and possibly antiatherogenic properties.9-15 and its deficiency has been implicated in the pathogenesis of hypertension, atherosclerosis, and preeclampsia.16-21 Nitric oxide is produced by the enzyme nitric oxide synthase (NOS) during the oxidation of the amino acid substrate L-arginine to L-citrulline.22 There are three isoforms of nitric oxide synthase (NOS), namely inducible NOS, endothelial NOS (eNOS), and neuronal NOS.23 The eNOS-derived NO is mainly responsible for maintaining vasomotor tone24. The eNOS is constitutively expressed by vascular endothelium, and its gene is assigned to chromosome 7. eNOS intron4 which is 27 base pair length can have 4 repeats (a allele) or 5 repeats ( b allele ). The VNTR polymorphism in intron4 of eNOS (eNOS4b/a polymorphism) has been reported to be significantly associated with the plasma NOx concentration26 and affects the transcription efficiency in a haplotype-specific fashionin linkage disequilibrium with the T-786C polymorphism in the promoter region.27 Nitric oxide has a very short plasma half-life of 6-60 seconds28 and within 10-20 minutes it breaks down rapidly into the stable products nitrate and nitrite29 and excreted through kidney. Upon coming into the bloodstream, nitrite reacts immediately with oxyhemoglobin to form methemoglobin. Consequently, most NO produced is detected in serum as the remaining product, nitrate30 and measurement of serum nitrite alone is meaningless. One of the most commonly used methods to measure serum nitrate is based on the reduction of nitrate to nitrite by cadmium or nitrate reductase, the nitrite produced being determined by Griess reaction. In this study eNOS phenotype analysis was done by measuring NOx (serum nitrate + nitrite) by cadmium-based reduction followed by griess method. In view of this in the present study we have evaluated the distribution of eNOS intron4 VNTR polymorphism among healthy south Indian (Tamil) individuals and the concerned phenotype (serum NOx level as an index of eNOS activity) was analysed using chemical method.
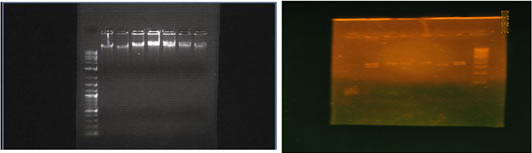
MATERIALS AND METHODS STUDY POPULATION The study sample comprised 282 unrelated south Indian people (male, female) of Mean age 50.59 + 10.52 years. Patients with hypertension, diabetes mellitus, and any chronic and acute illness were excluded from this study. Only those who smoked occasionally (frequency less than once a month), not smoked for past 2 months and non-alcoholics were included in the study. METHODS Resting blood pressure was recorded on each subject after a thirty minutes rest on a couch. Height and weight were recorded and blood samples were collected by Venipuncture after fortnight fasting in two test tubes. 2 ml of sample taken in EDTA-NaF tube was centrifuged at 2000 rpm for twenty minutes to get the buffy coat for DNA extraction and plasma used for sugar estimation. Another 3 ml of blood was taken in a plain tube, centrifuged at 2000 rpm for 20 minutes and serum was separated for serum NOx estimation. Plasma glucose level was estimated by GOD-POD method. Serum eNOS activity measured indirectly by reduction of serum nitrates to nitrites using copper activated cadmium granules and estimating total nitrites (NOx) using griess reagent. eNOS intron4 Polymorphism Screening DNA was extracted from buffy coat by high salt method37 and was used to amplify the intron4 sequence of the eNOS gene by PCR using primers sense 5′-AGGCCCTATGGTAGAGCCTT-3′and antisense 5′-TCTCTTAGTGCTGTGGTCA C-3′. Genomic DNA (1μg) was amplified in 25μl reaction mixture containing 0.3μmol/L of each primer and red dye master mix (Bangalore Genei) containing 100μmol/L of each dNTP, 2.5μL of 10x reaction buffer and 0.6 unit of Taq DNA polymerase. After the DNA was denatured for 5 minutes at 94oC, the reaction mixture was subjected to 30 cycles of denaturation for one minute at 94oC, 1 minute of annealing at 54oC and 1 minute of extension at 72oC. Final extension was carried over at 72oC for 10 minutes. eNOS intron4 polymorphism was detected by electrophoresing the PCR amplified product in 3% agarose gel and visualized under ultraviolet light after ethidium bromide staining. The PCR product is a 393 bp fragment in the presence of ‘a’ allele, and a 420 bp fragment in the presence of a ‘b’ allele. Thus, each DNA sample revealed one of the three possible patterns after electrophoresis: a 420 bp band (b/b genotype), a 393 bp band (a/a genotype), or both 420 and 393 bp bands (a/b genotype). Analysis was done using a 100 bp DNA ladder from Bangalore genei. Extracted DNA (lane 2 to 8) was tested on 1% agarose gel using 1kb ladder (lane 1) Ladder shows 10000, 8000, 7000,6000, 5000, 4000, 3000, 2000, 1000 bp fragments. (Figure.1) Genotype analysis done on 3%agarose gel using 100 bp DNA ladder obtained from genie (lane 8). Lane 6 shows one fragment corresponding to 393bp length – so the person is homozygous for a (aa), Lane 2,7 show two fragments – 393,420bp – Heterozygous (ab). Lane 1,3,4,5 one fragment – 420bp in length – homozygous (bb)(Figure.2) PHENOTYPE ANALYSIS eNOS activity was measured as serum NOx level using the following method. Step 1: Deproteinisation36- 300 µL of serum by adding 250µL of 75 mmol/L ZnSO4 solution, stirring, and centrifuging at 10 000g for 1 minute at room temperature, after which 350µL of 55 mmol/L NaOH was added. Again, the solution was stirred and centrifuged at 10 000g for 3 minutes and the supernatant wasrecovered (free of turbidity). We diluted 750 µL of supernatant with250 µL of glycine buffer (45 g/L, pH 9.7). Step 2: Activation of cadmium - Cadmium granules were rinsed three times withdeionized distilled water and mixed in a shaker gently in a 200 mmol/L CuSO439 solution in glycine-NaOH buffer (15 g/L, pH 9.7) for 5 minutes till the color of the solution fades. The solution drained off and the step repeated for another time. The copper-coatedgranules dried in tissue paper and are to be used within 10 minutes. After use, the granulesare rinsed and stored in 100 mmol/L H2SO4 solution; they canbe regenerated by repeating these steps. Step 3: Reduction of nitrate - The nitrite and nitrate calibrators were diluted with glycine bufferjust as the serum samples were. Calibration curves were made overa linear range of nitrite between 0 and 100 µmol/L. freshly activated cadmium granules (2–2.5 g) were addedto 1 mL of pretreated deproteinized serum and calibrator. After continuous stirring for 10 minutes, the samples were transferredto appropriately labeled tubes for nitrite determination. Step 4: Nitrite assay. Nitrite was estimated by Griess reaction Reagent 1 consisted of 50 mg of N-naphthylethylenediamine dissolved in 250 mL of distilled water. Reagent 2 was prepared by dissolving5 g of sulfanilic acid in 500 mL of 3 mol/L HCl. Both solutionsare stable for at least a year at 4 °C. From the above tubes 200 L of sample were placed into fresh glass tubes. To it 800 L sulfanilamide solution were mixed in, followed by 750 L NED solution. We then waited for 10 min at room temperature for a pink colour development and absorbance was read at 545 nm within 60 min. the measured OD was plotted on the standardization graph and concentration found out.
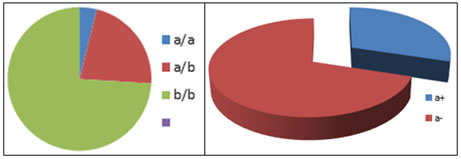
RESULTS The distribution of variables among the study population is represented in Table 1, 2 and 3. Gene frequency is shown in pie chart (Figure 3). Allele frequency is shown in pie chart (Figure 4). Age, Sex, BMI, lipid profile, blood sugar and conventional risk factor distribution among a+ and a- allelic groups were shown in Table 4. Since all the confounding factors were matched there were no significant differences between the two groups. Table 5 shows the comparison of eNOS activity (NOx level) among a+ and a- . Significantly low eNOS activity could be observed among a+(10.829 and SD-0.384) when compared to those with a- allele. (18.16 and SD 0.40) with P value of 0.011. Table 6 shows the difference in eNOS activity between different genotypes The activity was significantly lower among aa and ab genotype individuals when compared to bb genotype individuals. Table 1: Distribution of variables among the study population
TABLE 2: Genotype distribution of human eNOS intron 4 gene among the study population
Table 3: Allele frequencies of human eNOS intron 4 gene among the study population
Table 4: Characteristics of Patients With a+ and a- allele.
*Significant at P<0.05 ** Highly Significant at P<0.01 ***Very high Significant at P<0.001 Table 5: Comparison of eNOS activity among a+ and a- allelic groups:
*Significant at P<0.05 ** Highly Significant at P<0.01 ***Very high Significant at P<0.001
Table 6: Correlation between phenotype and genotype. serum NOx
*Significant at P<0.05 ** Highly Significant at P<0.01 ***Very high Significant at P<0.001 Figure 1 L1 2 3 4 5 6 7 8 L1 2 3 4 5 6 7 8 Figure 3 Figure 4: Allele frequency
DISCUSSION Genetic factors in combination with a number of environmental risk factors are involved in endothelial dysfunction which is hallmark for many chronic disorders like hypertension and atherosclerosis. Nitric oxide, the one considered to be the main factor involved in endothelial dysfunction, is found to be the main mediator involved in shear stress. The effect of physiological vasodilators like acetyl-choline, bradykinin and various therapeutic vasodilators were found to exert their action mainly via NO pathway. The lowered plasma NO level may be attributed to various factors like reduced biosynthesis and inactivation by free radicals. If it is due to reduced biosynthesis, it may be attributed to polymorphisms at the gene level, reduced transcriptional activity of eNOS gene, inactivation of eNOS and circulating eNOS inhibitors. Various studies are being conducted to find the role of endothelial nitric oxide synthase gene in regulating plasma NO level. The eNOS gene is regulated by various factors from the level of transcription to mRNA decay. Intron4 was found to affect the transcriptional efficiency of the gene and its polymorphism is being studied in relation to essential hypertension. This study was performed to seek for an apt report to find whether eNOS intron4 gene polymorphism is responsible for altered nitric oxide level. Those with impaired glucose tolerance, renal failure, acute infections, chronic inflammation and chronic smokers were excluded from the study as these states may present with altered serum NOx level. The main area of study was focused on serum NO level (NOx index) and eNOS intron4 polymorphism screening (genotypes aa, ab, bb). In the present study the frequency of ‘a’ allele was found to be approximately 0.27, which is little higher than that found in other populations viz., Iranian (0.1), Japanese (0.1 to 0.13), Turkish (0.14), Australian (0.17) and little lower than that of African Americans (0.28). The differences in the ethnic origin and sample sizes might have an influence in the results obtained regarding the distribution of the eNOS intron4 polymorphism studied in these populations. On comparison of the serum NOx levels between the various genotypes (a+ genotype and a- genotype), there was a significantly lower level among a+ (aa and ab ) genotypic hypertensives and controls with a p value was 0.011. This suggests that a+ genotype is significantly associated with low serum NOx level.
CONCLUSION The eNOS intron4 polymorphism may exert an effect on serum NOx levels, probably by altering the transcriptional efficiency. ‘a’ allelic persons were found to have lower level of serum NOx, when compared to ‘b’ alleles. When the eNOS activity was compared between a+ genotype and a- genotype there was a significantly low eNOS activity among a+ genotypic individuals (10.45) when compared to a- genotypic individuals (18.39). P value was 0.011, suggesting the fact that a+ genotype is associated with low eNOS activity and this low activity makes a person more susceptible to disorders like hypertension and atherosclerosis.
FUTURE PROSPECTS Other studies relating serum eNOS activity and antioxidant status have to explored. Various other eNOS genes have to be explored and their association with eNOS activity. Various transcriptional factors modulating the eNOS gene expression can be studied. Research aimed at identifying the strategies to improve eNOS activity can be performed.
REFERENCES
Policy for Articles with Open Access
|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 Home
Home