|
Table of Content - Volume 19 Issue 2 - August 2021
A correlative study of copper, iron, zinc and haematological parameters in oral cancer patients
Joel Sabu1, Ramlingareddy2*, Jayashree3, Malathi M4
1MBBS Student, 2Associate Professor, 3Tutor, Department of Clinical Biochemistry, S S Institute of Medical Sciences and Research Centre, Davangere, Karnataka, INDIA. 4Professor & HOD, Department of Clinical Biochemistry, Father Muller Medical College Mangalore, Karnataka, INDIA. Email: ramlingreddy2020@yahoo.co.in
Abstract Background: Alteration in levels of copper, iron and zinc variate the enzymatic actions in our body and thereby play a major role in etiopathogenesis of oral carcinogenesis. Aim of the study: The present study was mainly aimed to estimate, compare and correlate the serum levels of copper, iron and zinc with haematological parameters in oral cancer patients in comparison to normal controls. Methods: The collected serum of cases and controls were analyzed by using standard spectrophotometric methods in spectrophotometer analyzer and the data obtained was analysed and represented as Mean ± SD, mean difference was analyzed by Student’s T-test and Chi- square test for significance and strength of association by Karl Pearson’s correlation using SPSSv23 software. Results: Mean serum level of iron was significantly lower and the levels of zinc and copper in patients with oral cancerous lesions were significantly higher than that of healthy individuals. Pearson’s r data analysis, revealed a significant negative correlation between iron with platelets, and MCHC (Mean Corpuscular Haemoglobin Concentration). A significant positive correlation was seen between iron with haemoglobin and lymphocytes; between copper with leucocytes. Conclusions: The serum levels of zinc and copper in group I were significantly higher when compared to controls. There was a significant lower serum level of iron in Group-I when compared to controls. A significant correlation was seen in between serum trace elements and haematological parameters in oral cancer. This suggests the involvement of trace elements in variations of haematological parameters in the pathogenesis of oral carcinogenesis. Key words: Copper, Iron, Zinc, Oral cancer.
INTRODUCTION Copper, iron and zinc have inevitable roles in enzymatic action in our body.1-3 The cancer is so radical as it is the 6th most common cancer in the world.4 In 2020 there was an estimated 13,24,413 new cases of oral cavity and pharynx, and more than 8,51,678 deaths.5 Even though it is one of the most mortal cancers, the survival rate could be enhanced by quick diagnosis and referrals.6 Squamous cell carcinoma is the most prevalent form of oral cancer.7 So, to prevent various pathological manifestations and for early detections, variation in the levels of trace elements need to be treated. The development of cancer is a very complex process which takes a few years to get transformed from a pre-existing potential malignant lesion. Leucoplakia represents 85% of lesions that are potentially pre-malignant.8 Substance abuse, genetic mutations, chronic irritations and infections have ramifying effect in the aetiology of oral cancer.9 Since trace elements have a vital role in anti-cancerous activity and enzymatic reactions, its variations can lead to precancerous lesion and oral cancer. This is so because of the ability to counteract the oxidative stress induced by free radicals which cause serious damage cells in oral pre-cancerous conditions. So, it plays an indispensable role in diagnosis, treatment and prognosis as disease progresses.10However, the incidence of abnormal trace element status in oral cancer patients has not been comprehensively studied. Therefore, a study was conducted to evaluate the possible correlation in serum levels of copper (Cu), zinc (Zn) and iron (Fe) levels in oral cancer patients with various blood components levels in clinically diagnosed oral cancer patients when compared to normal healthy controls.
MATERIAL AND METHODS Source of data It was an observational analytical cross-sectional study where blood samples were collected from oncology unit and analysis was done in Father Muller Medical College Hospital, Clinical Biochemistry Laboratory over a period of 3 months. The study was conducted after getting approval from the institutional ethics committee and informed consent was obtained from all the study participants. Total number of 60 subjects were included in the study (sample size was achieved by using the sample size calculator for the power of >80% and alpha value <0.05) (8). Sample and sampling technique The formula used for calculation of sampling size n ≥ ( Z1-α/2 + Z1-β )2 ( σ12 + σ22 /r ) /( µ1 -µ2 )2 n-sample size σ-standard deviation µ-difference between means α-error,β-error Inclusion criteria: Group I (Cases): Thirty, clinically diagnosed oral cancer patients (age: 30-90 years) admitted in oncology unit of Medical College and Hospital. Group II (Controls): Thirty, normal, healthy, age and sex-matched volunteers (age: 30-90 years) Exclusion criteria: People suffering from Chronic Kidney Diseases, Liver disease, diabetes, infections were excluded from the study. Pregnant women were also excluded from study. Collection of samples: Blood samples were collected with aseptic precautions as per requirement and processed accordingly. The samples were collected from Clinical Biochemistry and Haematology Laboratory and stored in Deep Freezer for the purpose of study. Blood drawn for some other purpose was used both in cases and controls. Assays to be done: Following Biochemical parameters were analyzed using standard spectrophotometric methods in spectrophotometer analyzer.
All haematological parameters (Haemoglobin, RBC, WBC, Platelets, MCV, MCHC, MCH) were analyzed by using Beckman Coulter analyzer. Statistical Analysis: Collected data was represented as Mean ± SD, mean difference was analysed by Student’s T-test, Chi- square test for significance of difference and strength of association by Karl Pearson’s correlation, using SPSS v23 software.
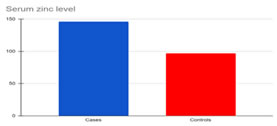
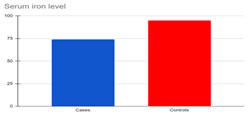
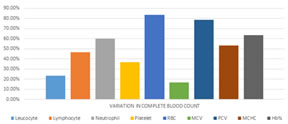
RESULTS Results of this study were presented in (Tables 1,2) and (Graphs 1,2,3,4). The subjects of the study were patients with oral cancer. (Group I; n = 30) and normal healthy controls (Group II; n=30). The serum levels of zinc and copper in group I were significantly higher when compared to controls. There was a significant lower serum level of iron in Group-I when compared to controls. Pearson’s r data analysis, revealed a significant negative correlation between iron with platelets, and MCHC in group I. A significant positive correlation was seen between iron with haemoglobin and lymphocytes; between copper with leucocytes in group I.
Table 1: Serum Levels of Iron, Copper and Zinc in both groups
*Significance of difference when compared to controls, p <0.05
Graph 1: Serum Zinc levels in Cases and Controls Graph 2: Serum Copper levels in Case and Controls
Graph 3: Serum Iron levels in Cases and Controls Graph 4: Variations in complete blood count in oral cancer patients
Table 2: Correlation of serum trace elements w.r.t haematological parameters in oral cancer patients
DISCUSSION The rate at which oral cancerous lesion spreading like an epidemic is alarming. The oral cancer lesion provides useful clinical markers for its diagnosis. Areca nut chewing is one of the most common causes for oral cancers. (14) The aetiology of oral cancer includes various carcinogens in tobacco and related products such as polynuclear aromatic hydrocarbons and nitrosamines.15The present study made an attempt to compare and correlate serum levels of copper, iron and zinc with other haematological parameters in patients with oral cancer and controls. A significant negative correlation was seen between iron with platelets and MCHC. A significant positive correlation was seen between iron with haemoglobin and lymphocytes; between copper with leucocytes were seen. The present study observed significantly lower serum iron levels in group-I when compared to controls. However, Baharvand et al.,16 observed that serum iron levels decreased in contrast to the present study. Guruprasad R et al.,18 attributed that decreased serum levels of iron was due to its utilisation for collagen synthesis. The serum zinc and copper levels in group I patients were significantly higher compared to controls. Even though the reason behind an increase in serum levels of copper is not known, it might be due to decreased catabolism of serum ceruloplasmin or due to the increased production of ceruloplasmin by liver due to inflammatory response by liver.17 Franklin RB et al.,19 attributed that increased zinc levels might be due to their important role in the rapid cell proliferation as encountered in growing tumors. There was a significant positive correlation between iron and haemoglobin in comparison to study done by Busti F et al.,20 because of its vital role in synthesis of haemoglobin. The study does not show any correlation between hemoglobin and copper in comparison to study done by Hegazy AA et al.,21 There was a significant positive correlation between iron and lymphocyte in comparison to the study done by Mascotti DP et al.,22 observed that iron is a very important element in the proliferation of lymphocytes. Whereas, non-significant positive correlation between zinc and lymphocyte was observed in the study. There is a significant negative correlation between iron and platelets in comparison to study done by Evstatiev R et al.,23 attributed that iron deficiency had increased commitment of hematopoietic progenitors to megakaryocyte lineage, accompanied by accelerated megakaryocyte differentiation.23 There was a significant negative correlation between iron and MCHC in comparison to study done by Bhattathiri et al.,24 attributed that there was an increase in MCHC due to compensatory regenerative attempts by bone marrow and a decreased levels of iron due to its usage by bone marrow and tumor. A significant positive correlation was seen between copper with leucocyte in comparison to the study by Shoaib M et al.,25 attributed that copper, being a cofactor for various enzymes, was directly linked with the synthesis of leucocyte.25
CONCLUSION The present study has made an attempt to estimate and correlate serum trace element levels in oral cancer patients with various blood components and their levels. There was significant correlation with various haematological parameters, which was evident from the study findings. The study suggests the involvement of oxidative stress, trace elements in altering the haematological parameters and in the etiopathogenesis of oral carcinogenesis. Future studies with larger sample size in assessing the biomarkers for prognosis and treatment follow up of oral cancer is required.
Acknowledgement To all authors and Father Muller Medical College and Research Centre for encouraging, funding and supporting the research.
REFERENCES
Policy for Articles with Open Access
|
|
 Home
Home