|
Table of Content - Volume 20 Issue 1 - October 2021
Evaluation and characterization of melatonin conjugates as an immunogen
Lathika1,2, Manupriya B R3, Tanhaji Ghodke4, V B Kadwad5, K Bhasker Shenoy6, H M Somashekarappa7, Shrikant L Patil8*
1,8Department of Physiology, K. S. Hegde Medical Academy, Deralakatte, Mangalore, Karnataka, INDIA. 2,3,4,6Department of Applied Zoology, Mangalore University, Mangalore, Karnataka, INDIA. 5Radiopharmaceuticals Programme, Board of Radiation and Isotope Technology (BRIT), Vashi, Navi Mumbai, Maharashtra, INDIA. 7Centre for Application of Radioisotopes and Radiation Technology, Mangalore, Karnataka, INDIA. Email: shrikantlpatil@gmail.com
Abstract Background: Melatonin is an indoleamine secreted by the pineal gland, and it is involved in circadian regulation, facilitation of sleep, inhibition of cancer development and enhancement of immune functions. Due to its low molecular weight, melatonin is considered a very weak immunogen. In order to generate antibodies against melatonin, it was conjugated with a high molecular weight protein (BSA and OVA). Materials and Methods: Melatonin is conjugated with BSA and OVA by a Mannich coupling reaction. Formaldehyde was used to couple the melatonin to the amine group of BSA/OVA via methylene or (CH2-) as a linker group. The conjugates were characterised using spectrophotometric analysis, titration with Trinitrobenzene sulfonic acid (TNBSA) and gel electrophoresis methods. Results: Spectrophotometric analysis showed that the range of melatonin incorporated to BSA is 54% and OVA is 19%. The conjugation efficiency of BSA and OVA, calculated by titrating conjugates with TNBSA, were 46% and 23%, respectively. Conclusions: The characterization of melatonin results showed that BSA is a better carrier protein than OVA for melatonin to exhibit potent immunogenic properties. Keywords: Melatonin, Conjugates, BSA, OVA,
INTRODUCTION Melatonin (N-acetyl-5-methoxytryptamine) is the primary hormone secreted by the pineal gland, and it is an essential indoleamine with an active role in regulating a robust circadian rhythm. Melatonin has a chemical formula of C13H16N2O2 and a molecular mass of 232.278 g/mol, determined by the X-ray diffraction method and confirmed by mass spectroscopy.1 Since melatonin is too small to produce antisera on its own, it must be coupled to an antigenic protein. Indole alkylamines have in common ring nitrogen (position 1) and an adjacent carbon (position 2). Thus, for melatonin, coupling through position 1 or position 2 should allow resulting antibodies to discriminate various indoles that are commonly present in tissues.2 A fundamental characteristic of an immunogen is that the number of haptens covalently attached to the carrier protein can be determined from the new molecular weight of the modified protein. It is crucial to characterize the prepared hapten-protein conjugates to determine the hapten density on carrier protein. The higher density of hapten increases the strength and specificity of the immune response. However, there is a risk that a high degree of substitution could adversely affect the activity and specificity of antibodies produced.3 Sometimes, the formations of protein-hapten conjugates may not be reproducible, even though prepared in the best experimental conditions. This may result in inconsistent hapten-protein stoichiometries resulting in considerable variation in desired antibodies generation. Various other methods have been used to determine the extent of hapten incorporation. By evaluating the available free amino groups before and after conjugation, hapten density can be determined spectrophotometrically.4 Due to its low molecular weight, melatonin is considered a very weak immunogen. In order to generate antibodies against melatonin, it was conjugated with a high molecular weight protein (BSA and OVA).
MATERIALS AND METHODS Materials: The study was conducted in Centre for Application of Radioisotopes and Radiation Technology, Mangalore, Karnataka, India. Melatonin, Ovalbumin (OVA), Bovine Serum Albumin (BSA), Formaldehyde, dialysis membrane-70, 2,4,6-trinitrobenzene 1-sulfonic acid (TNBS) were purchased from HiMedia Laboratories, Mumbai. All other chemicals and reagents used in this study were of high purity analytical grade. Buffers were made in Milli-Q double distilled water. Methods Melatonin conjugates were prepared by using 2 different carrier proteins as mentioned below: Mel-BSA conjugate: Melatonin was dissolved in 33% ethanol and is mixed with BSA dissolved in distilled water. Then acetate buffer (3M, pH-6.5) and formaldehyde were added. The reaction mixture was stirred and incubated for 20 hours in a dark room at room temperature] followed by 24 hours dialysis against distilled water.5 Mel-OVA conjugate: OVA dissolved in distilled water melatonin is dissolved in distilled water: ethanol mixture (2:1) were mixed. Sodium acetate buffer (3M, pH-6.5) and 7.5% formaldehyde were added. The reaction mixture was incubated in dark place with constant stirring for 24 hours followed by dialysis against 0.05M sodium phosphate buffer, pH- 7.4, overnight. The reaction product was finally filtered through 0.45µm filter unit6
Characterisation of Conjugates: Spectrophotometric analysis: UV absorbance spectrum method: Conjugates, non-modified BSA and OVA (1mg/ml in distilled water) were scanned from 200-500nm on a UV spectrophotometer.7 Optical Density measurement method: 5mg each of melatonin-BSA conjugate, melatonin-OVA conjugate, melatonin and BSA were dissolved in water and sulphuric acid was added incubated for 3 hours in room temperature.8 TNBS titration analysis: TNBS titration analysis: TNBS titrations were carried out by adding BSA or conjugate solution (1mg/mL) in a glass vial, 4% sodium bicarbonate and 0.1% TNBS solution. The vials were incubated for 2 hours at 37ºC in a water bath, and then 10%SDS solution was added followed by 1 M HCL. UV absorbances were measured at 342nm.9 Gel Electrophoresis: The SDS-PAGE of the conjugates were performed using Bio-Rad electrophoresis apparatus. Solutions of the conjugates were prepared in SDS-PAGE sample buffer to 1mg/mL and heated to 100ºC for 5 min. Gels were loaded with 5µl of sample and run for 90 min at 120V. Upon completion, gel was stained with Coomassie blue followed by distaining.9
RESULTS Both BSA-Melatonin and OVA- Melatonin conjugates were prepared using Mannich reaction (Figure 1). The conjugation efficiency of BSA and OVA, calculated by titrating conjugates with TNBSA are 46% and 23% respectively.
Figure 1: Synthesis of Mel-BSA conjugate by Mannich reaction In this study, conjugates were characterized by the following three methods: Spectrophotometric analysis, titration with TNBS, and gel electrophoresis. The results of spectrophotometric analysis and TNBS titration were reported as percent modified free amines in BSA by the melatonin and showed in the Table 1.
Table 1: Characterization of BSA-Melatonin and OVA- Melatonin conjugates
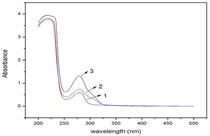
Spectrophotometric analysis of spectrum showed that the range of melatonin incorporated to BSA is 54% and OVA is 19% and from optical density calculation, it is 47% and 15% respectively. The UV absorbance spectrum for conjugates, BSA and OVA were obtained and showed in Figure 2. Figure 2: The absorption spectra of 1. BSA, 2. Mel-OVA, 3. Mel-BSA The characteristic absorbance contribution of Mel-OVA and Mel-BSA are observed at 278 nm and 279 nm, respectively, whereas that contribution of the carrier protein (BSA) is observed at 280 nm. Although equal amount of carrier protein and conjugates are taken, there is a difference in absorbance between conjugate and carrier protein, the absorbance of conjugates are higher than the carrier protein. The clear bands observed in SDS PAGE confirmed the conjugation (Figure 3).
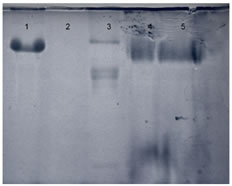
Figure 3: SDS-PAGE Gel image. 1. BSA, 2. Melatonin 3. OVA 4. Mel-BSA 5. Mel-OVA It was observed that the BSA molecules with higher conjugation density moved further, as this conjugate is more negatively charged in comparison to conjugates prepared with OVA.
DISCUSSION Earlier study has shown that melatonin antibodies are produced from the Mannich coupling reaction. It also highlights the melatonin to BSA structural compatibility, revealing a need to isolate the melatonin leads to a highly specific melatonin antiserum, as shown by radioimmunoassay cross-reactivity studies (RIA).2 The calculation of hapten density of prepared conjugate is crucial as it depends on the quality and quantity of antibodies produced. To generate a suitable antibody titre with higher specificity, an optimum number of haptens should be conjugated with a carrier protein. Higher substitution resulted in the release of more IgM than IgG and produced antibodies of lower affinity.10 It is concluded that high antibody titres with moderate antibody affinities are generally obtained with a hapten density of 15-30 molecules per carrier protein. A lower hapten density induces a slower immune response with higher affinity antibodies.11 Barbarakis and Bachas suggested using the synthesised conjugate's absorbance spectrum when the hapten has a strong chromophore that differs from the carrier protein.12 If the haptens chromophore is similar to the carrier protein, a differential spectrum obtained by subtraction of the non-conjugated protein spectra from the conjugated protein spectra method can be used. The available groups of surface lysine present in carrier proteins before and after conjugation were determined using TNBS reagent, a popular colorimetric method to determine the number of free amino groups in proteins and peptides. SDS-PAGE, which separates proteins based on molecular size, has been helpful when small proteins are conjugated with larger haptens. Although bands of conjugates yielded limited information about their molecular weight, its main advantage is in detecting cross-linked conjugates.7 The spectrophotometric method using TNBS and the UV spectrum were able to quantify the hapten-protein conjugation density accurately. From the results of our study, it can be concluded that BSA is a better carrier protein than OVA for melatonin.
CONCLUSIONS The characterization of melatonin conjugates as an immunogen result showed that Bovine Serum Albumin (BSA) is a better carrier protein than Ovalbumin (OVA) for melatonin to exhibit potent immunogenic properties. The BSA is a model protein, which has been conventionally used as a protein standard and in many areas of life sciences, biochemistry, pharmacology and medicine. Acknowledgements: We acknowledge the financial support by Board of Research in Nuclear Sciences (BRNS), Mumbai through Centre for Application of Radioisotopes and Radiation Technology (CARRT), Mangalore University, Mangalore. The authors are grateful to Mrs. Jayula Sarnaik, Mrs. Rani G. and Mrs. Shalaka Paradkar of the Radiopharmaceuticals Programme, Board of Radiation and Isotope Technology (BRIT), Vashi, Navi Mumbai, Maharashtra, India for providing the necessary laboratory and radiolabelling facilities.
REFERENCES
Policy for Articles with Open Access
|
|
||||||||||||||||
 Home
Home