|
Table of Content - Volume 20 Issue 2 - November 2021
Serum albumin levels in uncontrolled type II diabetes mellitus - An observational study
K Gunanithi1*, S Sakthidasan2
1Associate Professor, 2Professor, Department of Biochemistry, Melmaruvathur Adhiparasakthi Institute of Medical Sciences and Research [Affiliated to The Tamilnadu, Dr. M.G.R Medical University, Guindy, Chennai], INDIA. Email: gunamededu@gmail.com
Abstract Background: Type II Diabetes Mellitus, a common metabolic disorder characterized by hyperglycemia has the lethal potential to cause multiple pathologic and biochemical consequences. Albumin, the most abundant protein in plasma is synthesized by hepatic parenchymal cells. Several studies have found serum albumin level association with prediction, prognostication and complications of diabetes mellitus. The study was conducted to estimate the levels of serum albumin and to find its association with glycemic status among patients with uncontrolled type II diabetes mellitus in a Peripheral tertiary care institute – Adhiparasakthi Hospitals, Melmaruvathur Adhiparasakthi Institute of Medical Sciences and Research in Melmaruvathur, Tamilnadu during Jan-Mar 2020. The study includes 60 participants [ 30 cases and 30 controls], of both sexes aged between 40-60 years. Blood sample was analysed for fasting plasma glucose, serum total protein, serum albumin levels and serum creatinine levels. The Results of the study showed that the Fasting plasma Glucose levels were significantly [p<0.05] higher in Group 1 - Cases [177.63±44.45] when compared to Group 2 - Controls [91.76±11.77] and on the contrary Serum albumin levels were significantly [p<0.05] lower in Group 1 - Cases [3.12±0.58] when compared to Group 2 - Controls [4.10±0.42]. Pearson Correlation Coefficient between Serum Albumin and Fasting Plasma Glucose levels showed significant negative [r = - 0.609] correlation at 0.01 level [2-tailed]. The study concludes that Serum Albumin levels were significantly lower and has significant inverse association with Fasting Plasma Glucose levels in Uncontrolled type II Diabetes Mellitus patients with albumin levels being influenced by high plasma glucose levels through multiple mechanisms at various levels including synthesis, processing, glycation abnormalities, distribution, oxidative stress, and finally the degradation. Key Words: Type II diabetes mellitus, serum Albumin levels.
INTRODUCTION Type II Diabetes Mellitus, a common metabolic disorder characterized by hyperglycemia has the lethal potential to cause multiple pathologic and biochemical consequences. Around the World, its prevalence is constantly increasing all over. The number of people with diabetes rose from 108 million in 1980 to 422 million in 2014. Prevalence has been rising more rapidly in low income and middle-income countries than in high income countries. Between 2000 and 2016, there was a 5% increase in premature mortality from diabetes.1 In India,2 Diabetes is a growing challenge with estimated 8.7% diabetic population in the age group of 20 and 70 years. The prevalence of Type II Diabetes Mellitus is on the increase from 26·0 million in 1990 to 65·0 million in 2016. The prevalence of diabetes in adults aged 20 years or older in India increased from 5·5% in 1990 to 7·7% in 2016. In 2016, the Prevalence varied among States with highest in Tamil Nadu and Kerala and Delhi. Diabetes is a major cause of blindness, kidney failure, heart attacks, stroke and lower limb amputation. In 2019, an estimated 1.5 million deaths were directly caused by diabetes.1 Albumin, the most abundant protein in plasma is synthesized by hepatic parenchymal cells. It is the major contributor to colloidal oncotic pressure in the vascular space which helps to retain fluid within vascular space. The other functions of Albumin include binding and transport of many compounds including free fatty acids, bilirubin, calcium, thyroid and steroid hormones, drugs and maintenance of redox state of the extracellular milieu3. Serum albumin measurements are usually used in assessing liver disease as a part of liver function tests in routine biochemical analysis. Increased levels of albumin are rarely encountered in medicine practice but Hypoalbuminemia is a common clinical condition encountered in multiple diseases. Albumin is a negative acute phase reactant and inflammation is a common cause of hypoalbuminemia. Hepatic diseases, Urinary losses, GI losses, Burns, Protein energy malnutrition are other causes of hypoalbuminemia.4 Diabetes Mellitus, though a primary disorder of carbohydrate metabolism with abnormal glucose homeostasis, it also exerts consequences on lipid and protein metabolism. Several studies have found serum albumin level association with prediction, prognostication and complications of diabetes mellitus. The study was conducted to estimate the levels of serum albumin and to find its association with glycemic status among patients with uncontrolled type II diabetes mellitus.
MATERIALS AND METHODS The Study was conducted in a Peripheral tertiary care institute – Adhiparasakthi Hospitals, Melmaruvathur Adhiparasakthi Institute of Medical Sciences and Research in Melmaruvathur, Tamilnadu. It was conducted during Jan-Mar 2020 after obtaining formal approval from Institutional Research and Ethics Committee, MAPIMS. Informed Consent was obtained from all participants before the conduct of study. The Total sample size for the study includes 60 [ 30 cases and 30 controls], considering the minimum sample size calculation for statistical significance. The Study Participants includes adult males and females aged between 40-60 years attending General Medicine OPD [Cases] and Master Health Check-up [Controls] of Adhiparasakthi hospitals, Melmaruvathur adhiparasakthi institute of medical sciences and research, Melmaruvathur. The Mean age of Participants are 53.67±6.58 for Cases and 53.47±5.40 for Controls. Participant selection into the study was done by simple random sampling. Clinical history including Diabetic history, medications, duration and treatment follow up, Alcohol usage, diet history was obtained and baseline clinical examination was conducted before drawing blood sample. Inclusion criteria: Cases: Known Diabetic Mellitus5 Type II Patients [with/without treatment] for more than 5 years attending General Medicine OPD of Adhi Parasakthi hospitals. 5criteria for diagnosis of diabetes mellitus: Fasting plasma glucose ≥ 126 mg/dl [fasting defined as no caloric intake for atleast 8 hours] or 2 hour post glucose ≥ 200 mg/dl during OGTT. The test should be performed as described by WHO, using a glucose load containing the equivalent of 75 gm anhydrous glucose dissolved in water or HbA1c ≥ 6.5%. The test should be performed in a laboratory using a method that is NGSP certified and standardized to the DCCT assay. Or In a patient with classic symptoms of hyperglycemia or hyperglycemic crisis, a random plasma glucose ≥ 200 mg/dl. DCCT, Diabetes Control and Complications Trial; OGTT, Oral Glucose Tolerance Test; WHO, World Health Organization; NGSP, National Glycohemoglobin Standardization Program. In the absence of unequivocal hyperglycemia, diagnosis requires two abnormal test results from the same sample or in two separate test samples. Controls: Age matched Controls - Normal healthy volunteers attending Master Health Check of Adhiparasakthi hospitals. Exclusion criteria: Participants with known history of liver disease, renal disease, intestinal disease, malnourished individuals and alcohol abuse. Sample collection: Blood sample [6ml] was collected after overnight 8 hour fasting by venepuncture under strict aseptic precautions, 3 ml each in serum separator tube and in fluoride tube. Analytical methods: Blood sample was analysed for fasting plasma glucose as a measure of glycemic status, serum total protein, serum albumin levels and serum creatinine levels. Estimation of fasting plasma glucose was done by GOD-POD method, Serum total Protein by Biuret method, Serum Albumin by BCG method, Serum Creatinine by Jaffe method using Biosystems BA400 fully automated clinical chemistry analyzer in Central laboratory of Adhiparasakthi hospitals, Melmaruvathur Adhiparasakthi Institute of Medical Sciences and Research, Tamilnadu. Statistical Methods: The data were tabulated and statistical analysis which included student t test as test for significance and Pearson correlation coefficient as test for correlation was done using SPSS Statistical Package for Social Sciences version 18.
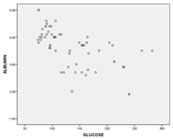
RESULTS AND DISCUSSION The Results of the study showed that the Fasting plasma Glucose levels were significantly [p<0.05] higher in Group 1 - Cases [177.63±44.45] when compared to Group 2 - Controls [91.76±11.77] (as illustrated in Graph 1) and on the contrary Serum albumin levels were significantly [p<0.05] lower in Group 1 – Cases [3.12±0.58] when compared to Group 2 – Controls [4.10±0.42] (as illustrated in Graph 2). Graph 1 Graph 2 Pearson Correlation Coefficient between Serum Albumin and Fasting Plasma Glucose levels showed significant negative [r = - 0.609] correlation at 0.01 level [2-tailed] (as illustrated in Graph 3) Graph 3 Albumin is a non-glycosylated protein of 585 amino acids. It is the most abundant protein in plasma accounting for more than 50% of plasma protein mass. Because of its high plasma concentration and medium size, albumin is the major contributor to colloidal oncotic pressure in the vascular space. This force helps to retain fluid within vascular space. Albumin is also the major protein component of most extravascular body fluids. Approximately 60% of total body albumin is found in the extravascular space. It is highly soluble in water because of its high abundance of charged amino acids. It has a net charge of-12 at neutral pH. Albumin is synthesized by hepatic parenchymal cells.3 The synthetic reserve is substantial and has been known to increase about three-fold above normal. The usual half-life of albumin is 15 to 19 days. Albumin has multiple functions including maintenance of colloidal oncotic pressure, binding and transport of many compounds including free fatty acids, bilirubin, calcium, thyroid and steroid hormones, drugs and maintenance of redox state of the extracellular milieu. Hyperglycemia has its effects on synthesis, redistribution, oxidative stress, protein glycation process, function and finally degradation of albumin. It affects circulating albumin concentration among patients with uncontrolled type II diabetes mellitus patients. Many studies have even concluded that lowered albumin concentration as a predictive risk factor for incidence of Diabetes Mellitus Type II which completes the vice versa effects of lowered albumin concentration and elevated plasma glucose levels among patients with type II diabetes mellitus.
Albumin levels and diabetes mellitus: In several studies,6-7 those patients with T2DM (type II diabetes mellitus) have lower circulating albumin compared to those without T2DM (type II diabetes mellitus). Serum albumin concentration may indirectly reflect a diabetic individual’s insulin reserve and thus serve as an indirect indicator of glycemic control.8-9 Studies like Schmidt et al.10 too suggested the lowered albumin levels in diabetic patients. Albumin synthesis and diabetes mellitus: Human serum albumin is one of most abundant plasma proteins and heavily glycated in diabetes. Albumin constitutes more than 50% of plasma proteins. Albumin levels in plasma are affected by factors such as diet, lifestyle, inflammation, disease, drugs etc.11 Hyperglycemia exacerbates beta cell dysfunction and depletes insulin secretory reserve.12 Diabetes mellitus may result in decreased hepatic albumin synthesis.13 Insulin is an important regulator of albumin synthesis. This has been corroborated by impaired liver production of albumin during insulin deficiency and a 10% increase in daily albumin synthesis after insulin infusion in diabetic individuals.14 Insulin has effects on the synthesis rates of liver proteins such as albumin and fibrinogen. In vivo in rats and in rat hepatocytes cultures, insulin increased albumin gene transcription and mRNA synthesis in a dose-dependent manner.15-17 In contrast, insulin deficiency decreased both albumin gene transcription and mRNA concentration with a resultant decrease of albumin synthesis. In diabetes, albumin synthesis and secretion is decreased due to insulin deficiency. Therefore, it is expected that albumin levels decrease in diabetes. In diabetes the circulating albumin level is depressed. A 30-40% fall in the rate of albumin synthesis in uncontrolled diabetic patients as well as in perfused livers of diabetic can be restored by in vivo insulin; one basis is a marked decline in transcription of albumin mRNA. The relative extra vascular distribution volume are likewise decreased about 35% in diabetics.18 Genetic basis has also been proposed by studies which proposed that Diabetes mellitus decreases albumin promoter activity19 Albumin levels and glycation status in diabetes mellitus: Albumin levels has also been affected by Glycated hemoglobin levels in type II diabetes mellitus patients. Any variation in levels of albumin may change the stoichiometry of glycation of other plasma proteins glycation.20 In diabetic patients, however, plasma albumin concentration has been reported to be inversely related with HbA1c levels, revealing a large proportion of poorly controlled diabetes in patients with lower plasma albumin concentrations. This inverse relationship may also be explained by the fact that poorly controlled type II diabetes has been associated with a further decrease in insulin production and secretion by the pancreatic β-cell.21-23 Albumin levels and insulin resistance in diabetes mellitus: Energy supply is an important determinant for albumin production. Serum albumin levels has been suggested to be associated with insulin resistance.24-25Reduced albumin levels association with higher percent fat and higher plasma glucose concentration were concluded by many studies. Reduced albumin is associated with adipose tissue infammation and predicts type II diabetes mellitus, and is consistent with chronic low-grade inflammation due to obesity in the development of type II diabetes mellitus.29 Several studies have reported that serum albumin was positively associated with metabolic syndrome or metabolic risk factors including lipid profile, Blood Pressure, and body mass index. These results suggest that serum albumin is associated with insulin resistance.26-28 Albumin levels and oxidative stress in diabetes mellitus: Serum albumin is vulnerable to reactive oxygen species. The possible sources of oxidative stress in diabetes mellitus includes auto-oxidation of glucose, shifts in redox balances, decreased tissue concentrations of low molecular weight antioxidants, such as reduced glutathione and vitamin E and impaired activities of antioxidant defence enzymes such as superoxide dismutase and catalase. Hyperglycemia increases the production of reactive oxygen species (ROS) and decreases their scavenging by various mechanisms. Such an increase of ROS may disturb or modify various cellular functions and alter gene expression due to oxidative stress. High glucose induced mitochondrial overproduction of ROS and abnormal activation of NADPH oxidase, are thought to be the major sources of ROS associated with hyperglycemia, while sustained hyperglycemia may also decrease radical scavenging by manganese superoxide dismutase and the glutathione redox cycle.30 High glucose levels could stimulate cytochrome P450-like activity by excessive nicotinamide adenine dinucleotide phosphate-oxidase (NADPH) produced by glucose metabolism.31 Glycated albumin has aberrant ability to bind various ligands and acts as a precursor to advanced glycation end-products, leading to oxidative stress and inflammation. Moreover, there is evidence that glycated albumin (and other glycated proteins) elicits an immunological response that may further reduce albumin.32 Serum albumin is the most abundant serum protein whose redox modification modulates its physiological function, as well as serves as biomarker of oxidative stress. In vitro oxidation of amino acid residues leads to protein degradation, aggregation and cross-linking. Many studies33 showed the presence of elevated levels of oxidized albumin, in patients with diabetes mellitus type 1 and type II. Given the deleterious consequences of glycated albumin, it is not surprising that mechanisms to clear glycated albumin have evolved.34 Various studies have shown that Diabetes Mellitus is associated with increased formation of free radicals and decrease in antioxidant potential. This leads to oxidative damage of cell components such as proteins, lipids and nucleic acid.35 Albumin distribution-degradation and diabetes mellitus: Studies even showed the effect of Glycemic status on the relative distribution and degradation of albumin levels. Murtiashaw et al. showed that the Albumin degradation and relative extra vascular distribution volume are likewise decreased about 35% in diabetics.18
CONCLUSION The study concludes that Serum Albumin levels were significantly lower and has significant inverse association with Fasting Plasma Glucose levels in Uncontrolled type II Diabetes Mellitus patients. Albumin levels being influenced by high plasma glucose levels through multiple mechanisms at various levels including synthesis, processing, glycation abnormalities, distribution, oxidative stress, and finally the degradation. This concludes the vicious cycle of altered albumin and glucose homeostasis leading to metabolic consequences. It is thereby proposed that Uncontrolled type II Diabetes Mellitus patients be tested for the levels of serum albumin as a routine to detect early abnormalities and prevent the related consequences.
LIMITATIONS: Statistical limitations include small sample size, lack of logistic regression and multivariate analysis. The estimation of Glycated albumin levels is not done which would give additional information on the effects of glycation in uncontrolled type II diabetes mellitus patients.
REFERENCES
Policy for Articles with Open Access
|
|
 Home
Home