|
Table of Content - Volume 21 Issue 1 - January 2022
An observational study on the correlation of serum albumin levels with metabolic syndrome parameters in a peripheral tertiary care institute, Tamil Nadu, India
K Gunanithi
Associate Professor, Department of Biochemistry, Melmaruvathur Adhiparasakthi Institute of Medical Sciences and Research [Affiliated to The Tamilnadu Dr. M.G.R Medical University, Guindy, Chennai, INDIA. Email: gunamededu@gmail.com
Abstract Background: Metabolic syndrome also called syndrome X is a widely prevalent syndromic complication of multitude of diseases primarily including diabetes mellitus type 2 and other diseases including systemic hypertension and dyslipidemia. Albumin, the most abundant protein in the plasma synthesized by parenchymal cells of the liver. Serum albumin levels has been studied in the past among patients with diabetes mellitus for a causative association and for relationship association with various factors of metabolic syndrome including glycemic status, lipemic status and blood pressure status apart from obesity. Materials And Methods: An Observational Case Control study involving 60 participants [30 cases from diabetic outpatient department and 30 controls from master health check-up] conducted after formal IEC approval during January-march 2020 in Adhiparasakthi hospitals, Melmaruvathur, Tamil Nadu. 5 ml of whole blood was collected and analysed for various metabolic syndrome parameters including waist circumference, blood pressure, HDL cholesterol and plasma glucose levels along with serum albumin. The data obtained was tabulated and statistical analysis done using independent sample t test and pearson correlation using SPSS version 18. Results: The results of the present study showed that serum albumin levels were significantly lower in patients [1.643 ±0.271] gm/dl with metabolic syndrome when compared to controls [3.667±0.422] gm/dl and showed significant negative correlation (correlation significant at 0.01 level (2-tailed) with the parameters of metabolic syndrome including waist circumference (r=-0.529)(p=0.003), systolic blood pressure (r=-0.578)(p=0.001), fasting serum triglyceride levels (r=-0.626)(p=0.000), fasting plasma glucose levels (-0.931)(p=0.000) and significant positive correlation with fasting serum HDL cholesterol levels (r=0.638)(p=0.000) while correlation with diastolic blood pressure remained insignificant (r=-0.297)(p=0.111). Conclusion: The study concludes the lower serum albumin levels in metabolic syndrome patients having positive influence on antiatherogenic factors and negative influence on atherogenic factors which substantially contributes to the risk for future vascular events including cardiovascular and cerebro vascular events. Therefore, Serum albumin levels be measured as a part of metabolic syndrome workup for future risk assessment of cardiovascular events.
INTRODUCTION Metabolic syndrome also called syndrome X is a widely prevalent syndromic complication of multitude of diseases including primarily diabetes mellitus type 2 and other diseases including systemic hypertension and dyslipidemia. The prevalence of metabolic syndrome among adult population in India is 30%. The increasing prevalence of type 2 diabetes mellitus together with associated insulin resistance, increased intake of refined foods, altered diet patterns with increased fats, sedentary lifestyle additionally adds upto the burden of the metabolic syndrome in India1 Albumin, the most abundant protein in the plasma synthesized by parenchymal cells of the liver. It performs major functions like maintaining the colloidal oncotic pressure and transport of molecules in the blood. The normal reference range for serum albumin is 3.5-5 gm/dl.2 Abnormalities in levels of serum albumin are seen in many clinical conditions with increased concentrations found in dehydration and decreased concentrations found in many conditions, most commonly seen in hepatic diseases. Serum albumin levels has been studied in the past among patients with diabetes mellitus for a causative association and for relationship association with various factors of metabolic syndrome including glycemic status, lipemic status and blood pressure status apart from obesity. Many studies have shown relationship of serum albumin levels with metabolic syndrome3 while some studies have suggested otherwise.4 More so, the relationship studies have shown only for some parameters of metabolic syndrome, while other parameters have not. The importance of the present study is in finding out the relationship among serum albumin levels and all parameters of metabolic syndrome. The null hypothesis is serum albumin levels have no correlation with various parameters of metabolic syndrome. The aim of the study is to find the correlation of serum albumin levels with various parameters (waist circumference, blood pressure, serum triglycerides, serum HDL cholesterol, fasting plasma glucose) of metabolic syndrome.
MATERIALS AND METHODS The study was conducted during the month January-march 2020 in Adhiparasakthi Hospitals, Melmaruvathur, Tamil Nadu. It was approved by the institutional research and ethics committee and the study was conducted with prior informed consent from the study participants. The minimum sample size for the study calculated is 40 by the institutional statistician. Sampling was randomized with the study participants includes 30 cases and 30 controls, of both sexes aged between 50-60 years. Cases were inducted into the study from the diabetic outpatient department and controls were inducted from the master health check up section of the Adhiparasakthi hospitals. Clinical history relating to type 2 diabetes mellitus and general clinical examination of the study participants was done before the study. Inclusion Criteria: Cases: known diabetic mellitus type 2 patients with features of metabolic syndrome criteria attending diabetic outpatient department of Adhiparasakthi hospitals. Criteria for diagnosis of Metabolic syndrome5: Any 3 out of the following
Controls: age matched controls –normal healthy volunteers attending master health check of Adhiparasakthi hospitals. Exclusion Criteria: Participants with known history of nutritional, gastrointestinal, liver, renal diseases were excluded from the study. Sample Collection: 5 ml of blood sample was collected after 8 hour overnight fasting and 2 hours post prandial by venepuncture under strict aseptic precautions. Analytical and Statistical methods: The analyte estimations are done for serum albumin levels, fasting plasma glucose levels, fasting serum triglyceride levels, fasting serum HDL cholesterol levels. The principle behind estimations includes testing for serum albumin by BCG method, Fasting plasma glucose by glucose oxidase peroxidase method, fasting serum triglycerides by Glycerol phosphate oxidase peroxidase method, fasting serum HDL cholesterol by direct enzymatic assay method using biosystems BA400 fully automated clinical chemistry analyzer of the institutional central laboratory, Melmaruvathur Adhiparasakthi Institute of Medical Sciences and Research, Tamil Nadu. The data obtained from the study participants including waist circumference, systolic and diastolic blood pressure, serum albumin levels, fasting serum triglyceride levels, fasting serum HDL cholesterol levels, and fasting plasma glucose levels were tabulated and student t test was used for significance testing and pearson correlation coefficient for correlation testing using statistical software statistical package for social sciences version 18.
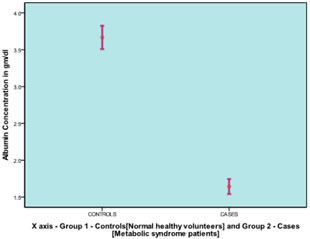
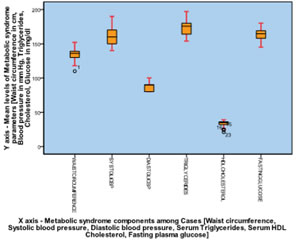
RESULTS The study results showed that serum albumin levels were significantly lower in patients [1.643 ±0.271] gm/dl with metabolic syndrome when compared to controls [3.667±0.422] gm/dl as illustrated in Graph [1] with mean waist circumference [134.60±9.71] cm, systolic blood pressure [159±14.22] mm Hg, diastolic blood pressure [89.33±6.91] mm Hg, fasting serum triglyceride levels [174.47±11.73] mg/dl, fasting serum HDL cholesterol levels [33.23±4.78] mg/dl and fasting plasma glucose levels [163.63±9.83] mg/dl noted among the cases as illustrated in Graph [2]. The correlation results of the study showed significant negative correlation (correlation significant at 0.01 level (2-tailed) with the parameters of metabolic syndrome including waist circumference (r=-0.529)(p=0.003), systolic blood pressure (r=-0.578)(p=0.001), fasting serum triglyceride levels (r=-0.626)(p=0.000), fasting plasma glucose levels (-0.931)(p=0.000) and significant positive correlation with fasting serum HDL cholesterol levels (r=0.638)(p=0.000) while correlation with diastolic blood pressure remained insignificant (r=-0.297)(p=0.111) as illustrated in Graph [3].
Graph 1 Graph 2 Graph 3 Graph 1: Mean comparison of albumin concentration among cases and control; Graph 2: Mean concentration of metabolic syndrome components among cases; Graph 3: Overlay scatter plot showing correlation between serum albumin levels and metabolic syndrome parameter among cases
DISCUSSION Metabolic syndrome: Metabolic syndrome, a constellation of various metabolic features involving altered glucose, altered lipid, altered sodium homeostasis. It has gained significance because of its association with atherosclerotic cardiovascular and cerebrovascular events.6 The primary component of the metabolic syndrome involves central obesity / visceral obesity which is measured by waist circumference and waist hip ratio. The association of other features including hypertension, central obesity, revolves around the basic alteration in general metabolic status. The pathogenic mechanisms of metabolic syndrome involves complex pathways with various factors involving diet, lifestyle, environment, physical activity playing major role. Insulin resistance plays a central role in pathogenesis of metabolic syndrome. It leads to lipolysis contributing to increase in circulating free fatty acid levels. It also contributes to hypertension because of opposing vasoactive action of insulin and free fatty acids.7 Visceral fat additionally contributes to insulin resistance and increased free fatty acid circulation. The increase in visceral fat increases leptin levels, an adipokine that controls energy homeostasis which is being altered in metabolic syndrome patients. It is also associated with reduced adiponectin levels.8 Activation of RAAS pathway also contributes to the development of hypertension, diabetes mellitus, dyslipidemia among metabolic syndrome patients.9 Hypoalbuminemia in Metabolic syndrome: The results of the present study showing significantly lower levels of serum albumin among metabolic syndrome patients shows the influence of various metabolic aspects affecting the albumin levels in the plasma beyond plasma glucose levels. Reduced albumin levels among metabolic syndrome patients are possibly due to multifactorial mechanisms involving major aspects like waist circumference, blood pressure, glycemic status, lipemic status of the metabolic syndrome with primary reason as insulin resistance. The correlation results of the study showing negative correlation for serum albumin with metabolic syndrome parameters including waist circumference, systolic blood pressure, fasting plasma glucose levels, fasting serum triglyceride levels and a positive correlation for serum albumin with serum HDL cholesterol levels while correlation remained insignificant for diastolic blood pressure. Decreased levels of serum albumin can be associated with various levels including albumin synthesis, distribution, half-life, transport function, vascular permeability, loss and degradation. Dietary factors play a major role in supplying essential molecules for albumin synthesis. Hormones like insulin has effects on the synthesis of liver proteins including albumin. It has been substantiated by in vivo studies on rats and rat hepatocyte cultures like Lloyd et al. which showed that insulin increased albumin gene transcription and mRNA synthesis in dose dependent manner and in contrast, insulin deficiency decreased both albumin gene transcription and mRNA concentration and resultant decreased in albumin synthesis.10-12 Insulin resistance in metabolic syndrome ultimately results in blocking the actions of insulin and thereby leads to low albumin synthesis leading to low serum albumin levels. Insulin resistance is a principal cause of type 2 diabetes and serum albumin has been associated with insulin resistance.13 Compensatory hyperinsulinemia seen in insulin resistance, predisposes to the development of metabolic impairments, including nonalcoholic fatty liver disease, IFG, and metabolic syndrome.14 This compensatory hyperinsulinemia may contribute to this relationship between insulin resistance and serum albumin levels. But the present study has not evaluated serum insulin levels in the participants which is a limitation of the study. Increased vascular permeability for cells and plasma solutes is universal in chronic diseases. In health states, FcRn [neonatal Fc receptor] which are the site specific mutants is bound to albumin and is found to prolong the half-life of albumin while there is substantial cycling into and out of the cells. In disease states, FcRn is downregulated which shortens the half-life of albumin.15-16 Oxidative stress and Inflammation are a part of chronic diseases including type 2 diabetes mellitus, dyslipidemia and hypertension. Stress and Inflammation have been described to contribute to reduced albumin synthesis. Hypoalbuminemia is associated with inflammation which is present in metabolic syndrome patients.17 Oxidative stress and chronic inflammation play crucial roles in generation of both insulin resistance and type 2 diabetes mellitus.18-19 Albumin has additionally been shown to have antioxidant and anti inflammatory properties which might well have been used up because of oxidative stress and inflammatory stress seen in metabolic syndrome patients contributing to hypoalbuminemia.20-22 Serum albumin concentrations were found to be associated with insulin resistance and fatty liver disease23 along with metabolic risk factors including lipid profile, blood pressure and body mass index.24 Visceral adiposity is the primary component of metabolic syndrome. The mechanisms of reduced albumin and weight gain particularly visceral adiposity is not fully understood but may have influence of appetite regulation. The binding capacity of albumin has been hypothesized to play a role in weight gain as albumin serves as a carrier protein for transport of various hormones and molecules, which ultimately influences the functions.25 Albumin-ghrelin binding has been shown to impair ghrelin biological activity. Reduced circulating albumin may result in less albumin-ghrelin interaction and thus greater ghrelin availability and activity of ghrelin stimulating appetite.26 Albumin also binds fatty acids which have been implicated as signaling molecules for hypothalamic appetite regulation.27 In steady state, the albumin synthesis rate be balanced by renal albumin clearance, Gastro intestinal albumin clearance and other catabolic components. The subclinical disease states of gastro intestinal system and renal system among metabolic syndrome patients might well be responsible for rapid clearance. Albumin is further glycosylated with increasing plasma glucose levels, and glycosylated proteins including albumin are rapidly cleared from the circulation by the liver.
CONCLUSION The study concludes that serum albumin levels are significantly lower in patients with metabolic syndrome and has significant negative correlation with major components of metabolic syndrome including visceral adiposity [waist circumference], diabetes mellitus [hyperglycemia], dyslipidemia [hypertriglyceridemia] and blood pressure [especially systolic blood pressure] and positive correlation with serum HDL cholesterol levels. Albumin levels are being lowered in metabolic syndrome patients due to multifactorial causes, with central adiposity and insulin resistance playing a major role in influencing the albumin levels at various phases including the synthesis phase, distribution phase, transport phase, catabolic phase of albumin homeostasis. This further accentuates the positive influence of albumin on antiatherogenic factors and negative influence on atherogenic factors which substantially contributes to the risk for future vascular events including cardiovascular and cerebro vascular events. Therefore, Serum albumin levels be measured as a part of metabolic syndrome workup for future risk assessment of cardiovascular events.
Limitations: Statistical limitations includes small sample size and the lack of logistic regression analysis. Technical limitations includes the lack of assessment of insulin levels which would have given the status of insulin resistance among the study participants. Lack of assessment of leptin, adiponectin and ghrelin levels which would have given the status of appetite regulation. A follow up on the albumin levels with the incidence study on vascular events on the study population would have added additional meaning in the role of albumin contributing to a safe role in prevention of such events among metabolic syndrome patients. Beyond the limitations, albumin has a role in general metabolism both in human health and in diseases.
REFERENCES
Policy for Articles with Open Access
|
|
 Home
Home