Official Journals By StatPerson Publication
|
Table of Content - Volume 4 Issue 2 - November 2017
Serum uric acid and urinary albumin in patients of type II diabetes mellitus
Gulajkar Supriya Rameshrao1, Bina M Gadhiya2*, A P Thorat3
1Assistant Professor, 2Associate Professor, 3Professor and HOD, Department of Biochemistry, Government Medical College and Hospital, Aurangabad, Maharashtra, INDIA. Email: gulajkarsupriya@yahoo.com
Abstract Diabetes mellitus has been known to be a state of oxidative stress with excess generation of free radicals contributed by several mechanisms including hyperglycemia. Uric Acid is the final oxidation product of purine metabolism in humans. Whether Uric Acid acts as an antioxidant or prooxidant is the matter of debate. Hyperuricemia is associated with an increased risk of complications. Increased levels could be injurious to the kidneys and predictive of progression of Renal disease. Objecives of study are to estimate Uric Acid levels in type II diabetics and study its correlation with urinary albumin excretion. Fasting venous samples along with urine were collected from patients visiting Govt Medical College, Aurangabad. Serum Uric Acid levels and urinary albumin were estimated. Uric Acid concentration was significantly higher in diabetics. Increased levels of uric acid were associated with increased urinary albumin excretion. The importance of uric acid as a risk factor for renal injury and increased urinary albumin excretion rate is worthy of further investigation. Key Words: Serum uric acid and urinary albumin.
It has been estimated that the global burden of type 2 diabetes mellitus (T2DM) for 2010 would be 285 million people (2010) which is projected to increase to 438 million in 20301. The burden of diabetes is to a large extent the consequence of macrovascular (coronary artery disease, peripheral vascular disease, and atherosclerosis) and microvascular (like retinopathy, neuropathy, and nephropathy) complications of the disease1. Diabetic nephropathy is a devastating late complication of diabetes in patients with both type 1 and 2 diabetes. Its early clinical sign is a slight elevation of urinary albumin excretion which is called microalbuminuria. If it progresses, patients will have progressive end stage renal disease (ESRD)3. There has been a growing interest in the association of hyperuricaemia with hyperglycemia since this was first described in 19234. Recently, renewed attention has been given to serum uric acid (SUA) because of its association with cardiovascular and renal disease and hypertension3. Hyperuricemia may increase the risk for renal dysfunction in patients with diabetes mellitus. Whether Uric Acid acts as an antioxidant or pro-oxidant is a matter of debate. So aim of our study is To estimate serum uric acid levels in type II DM To study correlation of uric acid with albumin excretion, Lipid profile and HbA1c in type II Diabetes Mellitus
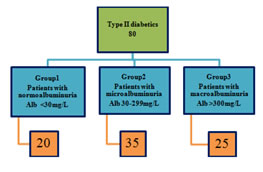
MATERIALS AND METHODS 80 type II diabetics (>5yrs) visiting Govt. Medical college, Aurangabad were included in the study. Informed written consents were taken. Cross sectional study was done.
Exclusion Criteria Alcoholics; Patients taking diuretics, uric acid lowering agents; Fever; Acute illness; UTI; Fasting venous samples were collected; Fresh midstream urine samples were collected for urinary albumin; Following parameters were assessed:
Table 1:
On the basis of albumin excretion patients were classified as Figure 1: RESULTS Demographic and biochemical characteristics of all the participants were analyzed as mean ± Standard Deviation (S.D.). ANOVA was applied to analyze the differences of studied characters in study groups. p value was obtained from ANOVA test as > 0.05 not significant, < 0.05 Significant and < 0.01 highly significant. Correlation coefficients (r) were calculated among various parameters in diabetic patients. Positive and negative r values were interpreted as follows: r = 0 (no correlation), r = 0 to 0.3 (poor correlation), r = 0.3 to 0.7 (considerable correlation) and r = 0.8 or more (strong correlation). Table 1: Demographic parameters (Mean ±S.D)
Table 2: Comparison of biochemical parameters in study groups (MEAN±SD)
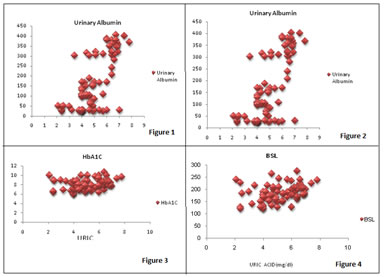
Uric acid levels are significantly higher in patients of Group 2 and Group 3 as compared to Group1. HbA1c,fasting BSL, lipid profile are significantly increased in patients of Group 2 and Group3 as compared to Group 1.
Table 3: Correlation of uric acid with other parameters
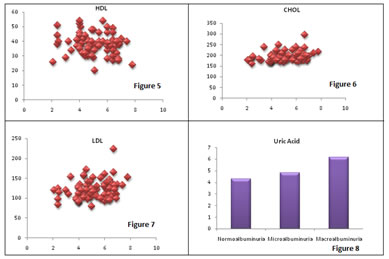
Uric acid levels were correlated positively with urinary albumin, fasting BSL, HBA1C, cholesterol, LDL. Negative correlation was found between uric acid and HDL. There was no correlation found between Uric acid and Triglyceride levels.
Legend Figure 1: Comparison of uric acid levels in study groups; Figure 2: Correlation of uric acid with urinary albumin; Figure 3: Correlation of uric acid with HbA1C; Figure 4: Correlation of uric acid with BSL; Figure 5: Correlation of uric acid with HDL; Figure 6: Correlation of uric acid with Cholesterol; Figure 7: Correlation of uric acid with LDL; Figure 8: Uric acid levels
DISCUSSION Study evaluated the relationship between serum uric acid and albumin excretion in type II diabetes mellitus. Albuminuria is an important marker of diabetic nephropathy in type II diabetics independent of hypertension. Hyperglycemia induced oxidative stress can alter endothelial permeability leading to albuminuria. Insulin resistance in type II diabetes is related to endothelial dysfunction increasing vascular endothelial cell permeability. Serum uric acid levels were significantly higher in Group 2 and Group3 as compared to Group1.Hyperinsulinemia which is the basis of type II diabetes may decrease uric acid clearance by kidneys and increase its production. In humans uric acid is the final breakdown product of Adenosine playing an important role in the insulin resistance. Adenosine can also cause increased renal uric acid retention. Uric acid levels were positively correlated with albumin excretion and correlation was significant. Reaction of xanthine oxidase in conversion of xanthine to uric acid generates superoxide anions. Endothelial dysfunction, one of the consequences of type II diabetes mellitus is thought to be mediated in part by such reactive oxygen species. Hyperuricemia induced endothelial dysfunction and renal hypertrophy decrease renal perfusion by stimulating afferent arteriolar vascular smooth muscle cell proliferation and alters vascular permeability causing albumin excretion. Hyperuricemia can induce renin expression from juxtaglomerular cells and inhibit NOS expression in macula densa. It also Stimulates cytokines from leukocytes and chemokines from vascular smooth muscle cells. Association of albumin excretion with uric acid confirmed the effect of hyperuricemia on diabetic nephropathy. Uric acid levels were also positively correlated with HbA1c and total cholesterol and LDL and correlated negatively with HDL. Lipid profile, HbA1C, were significantly increased in group 2 and group 3 as compared to group 1. Hyperglycemia and dyslipidemia cause disorders of the albumin excretion rate by damaging the podocyte and slit diaphragm protein scaffold with overproduction of and extracellular release of oxygen radical species at the glomerular level.
CONCLUSION Hyperuricemia is associated with diabetic patients and positively correlated with albumin excretion. Hyperuricemia is an independent risk factor for kidney dysfunction in patients with diabetes mellitus. Since it is relatively easy to lower uric acid levels with medications, this study suggested that not only the importance of uric acid as a risk factor for cardiovascular disease should be reconsidered, its pathogenic role of uric acid in other clinical manifestations such as renal injury and increased urinary albumin excretion rate is worthy of further investigation
REFERENCES
|
 Home
Home