Official Journals By StatPerson Publication
|
Table of Content - Volume 5 Issue 2 - February2018
Identification and corrective actions of preanalytical errors in clinical biochemistry laboratory of a pediatric tertiary care hospital: A two year study
Gouri Devi M1, Suresh Babu G2*, Abdullah Saad M D3, Suneetha R4, Rama Devi M5
1Associate Professor 2Assistant Professor, 3Resident Specialist, 4Biochemist, 5Professor, Department of Biochemistry, Niloufer Hospital, Hyderabad, Telangana, INDIA. Email: sushwasa@gmail.com
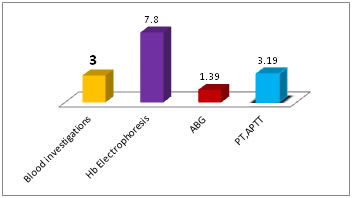
Abstract Objective: To identify the leading causes of preanalytical errors in clinical biochemistry laboratory and suggesting few corrections. Methods: A retrospective analysis of the records and log registers for 2016-17, for errors in the preanalytical phase at the clinical chemistry laboratory and this data was analyzed for the frequency of the main factors affecting the preanalytical quality of results. And correction measures are suggested accordingly. Results: Of the 168354 samples received, 61% were from pediatric ICUs and a total of 5120 i.e., 3% samples were found to have errors in the preanalytical phase. The error is more frequent for Hb electrophoresis samples (105 out of 1331 samples, i.e.,7.8%) because of clots, followed by coagulation samples(350 of 10945,i.e.,3.2%) for short draws. preanalytical errors in Transport and vacutainer categories predominated with 31% and 30% respectively. Out of 5120 specimens with preanalytics, 409 i.e., 8% of specimens, were unsuitable for further processing hence rejected (SR) due to wrong tubes (45%), clots (39%), clerical mistakes (12%) and broken tubes (4%). Conclusion: Identification and documentation with periodical auditing of preanalytical errors will confidently assure the quality and promote safety management of the patient. Key Words: Preanalytical errors; Quantity Not Sufficient QNS; Sample rejection SR. Total quality management TQM; Total testing process TTP;
Laboratory results play a significant role in patient management hence Total Quality management (TQM) is of utmost important one from any medical perspective. The total testing process (TTP) is the total process from the ordering of a test to the interpretation of a test result. The TTP starts and ends with the patient, and can sub divided into three distinctive phases: the pre-analytical step, the analytical step and the post-analytical step1. In the current scenario of automation and robotics, analytical as well as post analytical steps are under the control of a lab. But “preanalytical” phase is mostly, not under lab control; the one phase which encompasses multiple aspects and definitely a preventable part of lab errors. The preanalytical phase comprises all of the processes occurring before the sample is processed in the analyzer. These include inappropriate tests that have been ordered, improper sample collection, transport delays and illegible handwriting on requisition slips. Although these areas are beyond the jurisdiction of the clinical laboratory per se, the credibility of the labs is at stake due to these errors. As such, and according to reliable data, preanalytical errors still account for nearly 60%–70% of all mistakes occurring in laboratory diagnostics, most of them attributable to mishandling during collection, handling and preparing the specimens for testing2-7. Although most of these errors would be ‘‘intercepted’’ by laboratory professional or physicians before inappropriate actions are taken on otherwise unreliable results of laboratory testing, in nearly one-fifth of the cases these errors might be associated with further inappropriate investigations and unjustifiable increase in costs, and – even more notably – in 6.4% of the cases there might be a cause of inappropriate care or inappropriate modifications to therapy8,9. It is important for each laboratory to analyze of its own laboratory testing system to identify those areas where errors are likely to occur because each laboratory situation is somewhat different, and additional processes and sources of error may be identified. Once the processes have been documented, those that are most susceptible to error should be identified and should receive the most attention10. Our study has been conducted with the aim to determine nature and frequency of the occurrence of pre analytical errors and corrective measures suggested. The objectives formulated for present study were: to categorize, to determine the frequency of pre analytical errors; and to take corrective measures to prevent the occurrence of such errors in future.
MATERIALS AND METHODS This observational study is done from the retrieved data from the records of the period Jan 2016 to Dec 2017, i.e., for 2 years duration at the Laboratory Department of Biochemistry, Niloufer Hospital for women and children, Hyderabad, Telangana, India (is a tertiary care pediatric health care setup). Daily Internal quality controls and monthly external quality controls (EQAS) have been a routine in this laboratory to rule out any error in the analytical phase and assuming that any error that was there might be a pre-analytical origin. Fluids investigations (CSF, Urine and other fluids) have a complex preanalytics, hence were not included in this study and this topic is limited to the blood samples only. All the samples along with the requisition forms were carefully analyzed for any preanalytical mistakes and were noted in a register, which is a routine process at our lab reception. Sample rejection data with the pre analytical variable responsible was noted down in a register as a good lab practice (GLP). The unsuitable samples for testing process were recognized and identified, by a naked eye examination by the laboratory technician at the reception table as well as after centrifugation. Samples those have been rejected are stored in the laboratory for up to 24 hours at 4 °C, were immediately informed through the telephone to the concerned department stating that the sample has not been processed and if they can, to resend the sample as soon as possible. When precious specimens such as new born samples are received in unsuitable tubes or transported under inappropriate conditions, it is up to the on duty technical incharge or the lab physician who ever were available, to decide whether the sample should be processed with a preanalytical comment in the final report. The data was retrieved and summarized on monthly basis. A total of 168354 Blood samples which were received in the mentioned period at our lab reception were included for this research and analyzed accordingly. The distribution frequencies between the point of collection and the specimen rejections were evaluated by descriptive statistical analyses (Microsoft Excel).
RESULTS The laboratory received 1, 68,354 blood samples and preanalytical mistakes recorded were 5,120 (only 3% of total) samples, during 2016-17 (Table 1, Figure 1).
Table 1: Preanalytical errors according to the type of blood investigations and during the study period
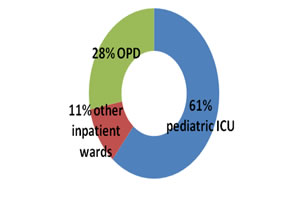
Out of the total number of error samples, 3123 (61%) were from pediatric ICUs, 563 (11%) from other inpatient wards and 1434 (28%) from outpatient services (Figure 2). Figure 2: Sample collection area wise preanalytics (5120 samples)
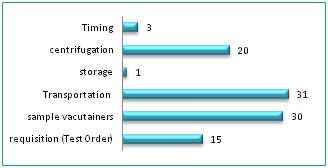
The preanalytical errors were categorized as A) Requisition (Test order); B)Sample vacutainers tubes; C)Transport; D)Storage, E) Centrifugation and F)Timing. Category wise potential problems were shown with their frequency as percentages in Table 2 and Figure 3. The individual quality indicators are shown in detail in Table 3.
Table 2: Preanalytical errors in general at our Lab
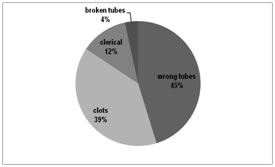
Out of 5120 specimens with preanalytical errors, 409, (8%) specimens were unsuitable for further processing hence rejected (SR) due to wrong tubes (45%), clots (39%), clerical mistakes (12%) and broken tubes (4%).
Table 4: Sample rejection in number
Table 3: Quality Indicators of the preanalytical phase and the outcomes with their frequency in% (*TAT: Turnaround time; SR: Sample rejection)
DISCUSSION The International Organization for Standardization (ISO) 15189:2012 standard for laboratory accreditation defines the preanalytical phase as “processes that start, in chronological order, from the clinician’s request and include the examination request, preparation and identification of the patient, collection of the primary sample(s), and transportation to and within the laboratory, and end when the analytical examination begins”11. For the prevention of preanalytical errors, the most reliable approach is to construct preanalytical standardization12. In our setup, bar-coding of the sample is not available hence; a request form generally accompanies the specimens. Re check is done for transcription errors by comparing results on the report forms with those on the results log book. Specimens that require further analysis because of dilution or assay problems are mentioned as “alerts” on the master log, as per the standard lab practices10. Each type of test has its own kind of preanalytical problem particular to that investigation. Some preanalytical errors are predominant in serum chemistry tests whereas those may not be important in others. For instance, hemolysis is a problematic error in serum potassium estimation but not for ABG, as the sample itself is a whole blood. An incomplete requisition can be corrected but a mismatched tube is rejected for ‘run’. Blood transfusion is a rejecting preanalytical error for Hb variants analysis but not for other serum investigations. In our laboratory, preanalytical errors were 3% for the blood tests which use serum as the sample. Insufficient volume (QNS) and the hemolysis were most common occurrences as the preanalytical errors (see Table 1, Fig 1). The manual agar gel Hb electrophoresis technique at our lab required at least 5ml of whole blood in an EDTA bottle, we often received improperly mixed sample giving rise to a clot sample. And QNS received, which were not suitable to prepare hemosylate and for fetal Hb testing. As these samples were drawn just before blood transfusions, it was not possible to re-draw the sample, hence sample rejected. Sometimes patient attendant reveals at the lab reception that patient was blood transfused a few days earlier before they took admission and thus the blood sample brought for Hb electrophoresis stands unsuitable. This particular preanalytical error also sometimes revealed retrospectively, i.e., post analytically, when the treating doctor consults the lab to discuss the results (for example, a possible false negative report; patient being positive for Sickling test but a negative for sickle Hb band in electrophoresis., as patient attenders conceal the fact of recent blood transfusion, for the fear of refusal of admission). For these reasons, preanalytical factors misguide the total testing process; hence out of 1331 specimens, 105 (7.8%) preanalytical errors occurred for this test. ABG analysis had 1.39% (20 of 1435) of storage and transport errors. The arterial blood samples were often brought without icepack and rarely, those were sent to the lab without being kept stored in a refrigerator. The noted preanalytical mistake for PT, aPTT samples were mostly a “short draw”, (3.19%) i.e., the blood was short of the mark given on the vacutainer tube (see Fig 5). This error results in improper ratio of blood to anticoagulant Tri Sodium Citrate and thus gives an erroneous result. We also received samples as clots because of improper mixing of blood. The guidelines from Lippi et al states that tubes should not be accepted for analysis if < 80 % full because the results of PT, APTT, thrombin time were significantly longer when tubes were under-filled, frequently to the extent that patient management decisions could be affected13. Samples which arrived from ICUs were having 61% preanalytical errors, followed by samples from OPD, 28% (Fig 2). We could replace the samples which had preanalytical errors, except from OPD, with the adequate and suitable samples from the respective wards. The preanalytical mistakes were rectified in most of the cases by communication to respective collection areas except for a few number of blood investigations. Pediatric /Neonatal ICUs were the problem area for our pre analytics as here, sample collection is a difficult task for the phlebotomists, with inevitable insuffient volume (i.e., QNS), in spite of their sincere effort to draw sufficient volume. OPD patients were non traceable due to lack of communication in many of cases. All the preanalytical errors were categorized in to 6 types (see Table 2, Fig 3) and transportation error category is a predominant observation (31%) as the samples were carried ‘by hand’ from respective wards and especially OPD, open to the environment which could subject the sample tubes to temperature changes and from one building to another leads to exposure to the sunlight and the distance by walk has resulted in delayed transport and delivery of the samples to laboratory reception. This could have resulted in erroneous bilirubin values. Vacutainer tubes category has 30% of preanalytical errors with QNS (25%), mislabeling on tubes and clot samples. Often, in precious samples, we had to dilute the available serum and ‘run’ the tests. Centrifugation of blood samples surfaced the preanalytical errors to 20% with hemolysis, fibrin clots and lipemic. Lipemia interferes with optical reading by the instrument and can affect interpretation of electrolyte values. Test order request slips category amounted to 15% of preanalytical mistakes which were mostly “clerical”. In house deliveries of twins, also given rise to preanalytical mistake; of single test requisition with single ID but accompanied twin samples. Timing of sample drawing is also a contributory factor, as patients force phlebotomists to take their sample before scheduled time of post prandial or before 2h time of OGTT. This inappropriately increases the incidence of lipemic samples in OPD patients, may be due to lack of information regarding prior preparation to the patients by the clinicians as well as ignoring of preparation rules by the patients. Hence, many patients give samples in non-fasting states leading to erroneous reporting14. Sample storage category contributed the least (1%) of preanalytical mistakes because those specimens reaching the lab are very few. These data are comparable to those provided by other investigators, which confirm that problems directly related to specimen collection are the main cause of Preanalytic errors, especially hemolysed, clotted, insufficient, and incorrect samples14,15,16.
Table 5: Corrective actions suggested improving various categories of preanalytical errors
Figure 5: A few examples are shown for preanalytical errors
Our lab specimen rejection rate was 8% see (Table 4 and Fig 4), comparable to a study done by Dikmen ZG et al [6]. But, QNS was our major concern (25%) leading to difficult decisions for ‘SR’ or to run test with dilution as 61% of total samples received were ‘precious’ from neonatal / premature born ICUs. Though clerical mistakes (12%) were corrected to a few extents, broken (4%) and wrong tubes (45%) were rejected. Fibrin clots (39%) were a second major factor for our rejection, pre or post centrifugation. Category wise quality indicators of preanalytical phase of total testing process and their individual outcomes are mentioned in detail in Table 3 and a few examples with images are shown in Figure 5. The Phlebotomy techniques used to acquire a specimen also affect many laboratory tests, hence this been considered a separate area of improvement for medical technicians in developed countries17,18. For example, prolonged tourniquet application causes local anoxia causes potassium leak from cells, and the back venous pressure concentrates cells, proteins, and substances bound to proteins (such as calcium). Monitoring by a QC specialist will minimize errors as detected by limit checks, delta checks, etc19. Creating awareness for accurate and timely submission of laboratory test request forms, training in the correct method of sample drawing (using evacuated tube system) and the distribution of protocols for proper specimen collection and handling, as well as periodical auditing of preanalytical mistakes is helpful and this can be used in a targeted manner for information and training purposes, providing the direct link between the blood collection practice and the resulting sample quality issues6,15. Lippi G, McBride KB et al, states that ‘preanalytical errors often cause random errors undetectable by normal quality control methods. In order to determine the cause of these random errors, it is necessary for the laboratory professional to become a sort of “detective”. Through a series of deductions and research, (s) he can identify the cause and put corrective actions in place’13. A few corrective actions are suggested for the preanalytics as well as to reduce SR to a minimum level is shown in a tabular form in the Table 56, 10,20.
CONCLUSIONS Every laboratory must identify and document the steps in total testing process; especially preanalytical errors part which is mostly not under the purview of the lab. Thus devising corrective strategies for their prevention can significantly reduce the laboratory errors and minimize sample rejection. At the same time quality reporting of a laboratory is assured and laboratory can confidently contribute to the safety management of the patients.
REFERENCES
|
 Home
Home