Official Journals By StatPerson Publication
|
Table of Content - Volume 6 Issue 3 - June 2018
Evaluation of thyroid function in patients of chronic kidney disease
V Ratnakumari1, P Kiranmai2*
1,2Associate Professor, Department of Biochemistry, Osmania Medical College, Koti, Hyderabad, Telangana, INDIA. Email: drkiranmai_23@yahoo.co.in
Abstract Background: Chronic Kidney Disease (CKD) is abnormal kidney function with low GFR (<60 ml/min/1.73m2) for 3 or more months. Prevalence of CKD in India is 17.2% and over 2 million people world wide receive dialysis treatment. Kidney plays an important role in the metabolism and excretion of thyroid hormones. CKD is known to affect the pituitary thyroid axis and the peripheral metabolism of thyroid hormones. Euthyroid state, hypothyroidism and hyperthyroidism have all been reported in previous studies. Considering the variation of thyroid function tests in patients with CKD in previous studies, the present study is undertaken to evaluate thyroid function and to establish a correlation if any between thyroid dysfunction and severity of CKD. Materials and Methods: 50 patients of established CKD in the age group of 30-60 yrs without thyroid disease were included in study group and 50 age matched healthy subjects formed the control group. Thyroid function is evaluated in both groups by measuring Total serum T4, serumT3 and serum Thyroid stimulating Hormone (TSH). Serum creatinine and blood urea are measured in both the study and control groups. Statistical analysis was done by student t test by comparing mean serum values. Results: In study group mean serum creatinine and mean blood urea level are higher than the mean blood urea level in control group, which is statistically significant (p<0.001). Mean value of serum T3 level was significantly less in study group (0.692±0.37) when compared to control group (1.19±0.82) (p <0.001). Mean value of serum T4 level was significantly low in study group (5.4±2.6) as compared to control group (7.86±1.94) (p<0.0001) Mean serum TSH level was significantly higher in study group (4.76±2.99) when compared to the control group (3.12±1.77) (p<0.001)).Patients with high TSH levels above normal range (0.34 to 5.6 µIU/L ) were 17. The prevalence of subclinical hypothyroidism is calculated as 34 % in study group. In control group only two subjects were having more TSH levels than normal range. So prevalence of subclinical hypothyroidism is 4 %. Conclusions: Mean serum T3and T4 levels are low and mean serum TSH levels are high in study group compared to control group suggesting association of subclinical hypothyroidism in cases of CKD. Key Words: Chronic Kidney Disease (CKD), Serum T3, Serum T4, Serum TSH.
INTRODUCTION Chronic Kidney Disease (CKD) is a public health problem affecting 5-10% of the world population1. Global burden of disease study in 2010 ranked CKD at 18th place in the list of causes of total number of deaths worldwide2. CKD is abnormal kidney function with low GFR (<60 ml/min/1.73m2) for 3 or more months3,4,5. Prevalence of CKD in India is 17.2% and over 2 million people world wide receive dialysis treatment6. Thyroid hormones T3 (Tri idoThyronine ) and T4 (Tetra iod Thyroxine) are the main regulating hormones of body metabolism. They have a significant role in the growth and development of the kidney for the maintenance of Acid base and electrolyte homeostasis7,8. Whereas kidney plays an important role in the metabolism and excretion of thyroid hormones. CKD is known to affect the pituitary thyroid axis and the peripheral metabolism of thyroid hormones9. Previous studies of thyroid function in CKD patients have shown conflicting results. Euthyroid state, hypothyroidism and hyperthyroidism have all been reported by various workers10,11,12,13,14. The incidence of goiter in CKD patients is also reported in literature15,16,17. A study has reported reversion of thyroid function to normal after successful renal transplantation13. Development of immunemediated glomerular injury has been linked to thyroid disorders18,19,20. Considering the variation of thyroid function tests in patients with CKD in previous studies, the present study is undertaken to evaluate thyroid function and to establish a correlation if any between thyroid dysfunction and severity of CKD.
MATERIALS AND METHODS The study was conducted in the department of Biochemistry Osmania General Hospital. Fifty (50) patients of Chronic Kidney Disease (CKD) admitted in the department of Medicine in the age group of 30-60 yrs formed the study group. Diagnosis of chronic kidney disease was established based on the clinical presentation with kidney disease of > 3 months duration. and biochemical tests like Serum creatinine and Blood urea. Exclusion Criteria: Patients with family history of thyroid disease, patients on medications like beta blockers and Corticosteroids were excluded from the study. Fifty (50) age matched healthy subjects without kidney and thyroid disease formed the control group. They were selected from the working staff of Osmania General Hospital. After taking due consent 12 hrs fasting venous sample of 5ml was collected from patients and subjects in both study and control groups. Thyroid function is evaluated in both groups by measuring Total serum T4, serumT3 and serum TSH by Chemiluminiscence Immunodiagnostic method on Seimens Advia Centaur. (Normal ranges Serum T3-0.87 to 1.78ng/ml, Serum T4- 6.09 to 12.23µgm/dl and serum TSH- 0.34 to 5.60 µIU/ml) Serum creatinine and blood urea are measured in both the study and control groups by creatininase and urease methods respectively (Enzymatic Kinetic photometric methods- Beckman coulter Autoanalyser AU 5800). Statistical Analysis: The results are analysed on SSPS statistical software. The estimates are expressed as Mean±SD and the comparison is done by using student t test. Statistical significance is indicated by p value < 0.05.
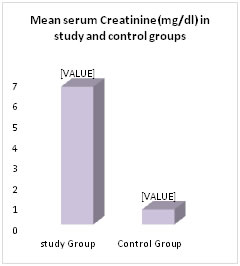
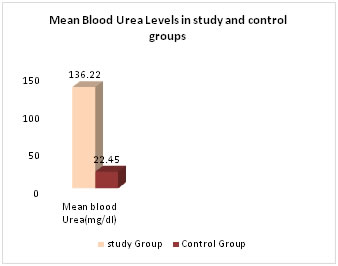
RESULTS 50 patients admitted in the department of Medicine with established diagnosis of CKD based on clinical and biochemical evaluation in the age group of 30-60 yrs formed the study group. (n=50). 28 were males and 22 were females. The control group included 50 healthy age matched subjects 34 males and 16 females. Serum creatinine, blood urea, serum T4, T3 and TSH are measured in both study and control groups. In study group mean serum creatinine is 6.68±3.48, this is higher than mean of control group0.73±0.24.which is statistically significant (p<0.001). Mean blood urea level in study group 136.22±48.36 is higher than the mean blood urea level in control group 22.45±8.56, Which is statistically significant (p<0.001) Table-1.Fig-1
Table 1:
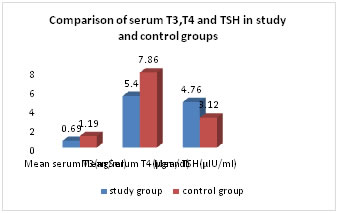
Figure 1: Comparison of serum creatinine and blood urea levels in study and control groups Mean value of serum T3 level was significantly less in study group (0.692±0.37) when compared to control group (1.19±0.82) (p <0.001) Table-2. Fig-2. Mean value of serum T4 level was significantly low in study group (5.4±2.6) as compared to control group (7.86±1.94) (p<0.0001) Mean serum TSH level was significantly higher in study group (4.76±2.99) when compared to the control group (3.12±1.77) (p<0.001)) Table-2. Fig-2.
Table 2: Comparison of Serum T3,T4 and TSH mean values in study and control groups
Figure 2: Comparison of Mean serum T3, T4 and TSH levels in study and control groups
DISCUSSION CONCLUSIONS REFERENCES |
 Home
Home