Official Journals By StatPerson Publication
|
Table of Content - Volume 7 Issue 2 - August 2018
A study of serum Cystatin C levels in type 2 diabetes mellitus patients with normoalbuminuria, microalbuminuria and macroalbuminuria
S Sindu Priya1*, S Uma Maheswari2
1,Assistant Professor, Department of Biochemistry, Annapoorana Medical College and Hospital, Salem, Tamil Nadu, INDIA. 2Assistant Professor, Department of Biochemistry, Govt. Coimbatore Medical College, Coimbatore – 14, Tamil Nadu, INDIA. Email: sindukarthick@gmail.com
Abstract Background: Diabetic nephropathy, a major microvascular complication of diabetes mellitus, is a leading cause of end-stage renal disease. Early detection of renal dysfunction is the only way to avoid the morbidity and mortality in type 2 diabetes mellitus(T2DM). Many studies have demonstrated that serum cystatin C is an accurate and sensitive marker for detecting early renal impairment in T2DM. Hence this study was done to assess the serum cystatin C as an early marker of diabetic nephropathy. Aim: To measure the serum cystatin C levels in T2DM patients with normoalbuminuria, microalbuminuria and macroalbuminuria. Materials and Methods: 90 known T2DM patients in the age group of 40-70 years were selected and further categorized according to the urine albumin-creatinine ratio into normoalbuminuria, microalbuminuria and macroalbuminuria, with 30 patients in each group. Height, weight, BMI and serum cystatin C were measured. Results: Mean of cystatin C was 0.94+0.23, 1.11+0.25 and 1.64+0.68 mg/L for the three groups respectively. In group A, group B and group C, the normal cystatin C levels were 70%, 56.7% and 20% respectively. Conclusion: Cystatin C is more sensitive, possibly a better marker for detection of early renal impairment in T2DM. Key Words: Normoalbuminuria, Microalbuminuria, Macroalbuminuria and Cystain C.
INTRODUCTION Diabetes mellitus is a group of metabolic diseases characterized by hyperglycemia resulting from defects in insulin secretion, insulin action, or both1. Diabetes, once considered as a disorder of elderly people, but now has become a major cause of morbidity and mortality in youth and middle aged persons. Many factors like population explosion, ageing, urbanization, obesity and physical inactivity are contributing to the increased prevalence of diabetes. Diabetes has become a major global health problem. This disease affects 6.6% (285 million people) of the world's population in the 20-79 years age group2. According to the International Diabetic Federation (IDF), this number is expected to grow to 380 million by 20253,4,5. In parallel with the increase in diabetes mellitus (DM), an increase in the number of patients with prevalence of one of its microvascular complications diabetic nephropathy has also been noted which has become the single most common cause of end-stage kidney disease6. Hence, it is very important to prevent or at least slow down the process of renal damage in diabetes mellitus. Microalbuminuria was described more than three decades ago as a predictor of nephropathy and associated with higher cardiovascular risk7. Once diabetic nephropathy develops, renal function deteriorates fairly rapidly and renal insufficiency develops8. Microalbuminuria is recognized as a sign of abnormal vascular function and increased vascular permeability9,10. It has also been considered as the first indication of renal injury in diabetes mellitus patients. Serum or plasma creatinine levels have become the most commonly used markers for GFR determination because of the simplicity and lower costs of this method11,12. However, serum creatinine concentration does not increase until renal function decreases to less than 50% of the normal value13. Serum creatinine is an insensitive indicator of diminished GFR because its concentration is affected by meat intake, gender, muscle mass, malnutrition and ageing14. Hence there is a need for a novel marker that detects early renal impairment, so that we can prevent the morbidity and mortality. Serum cystatin C, a cysteine protease inhibitor, which is freely filtered by the renal glomeruli and metabolized by the proximal tubule has been identified as a promising marker of renal impairment.
MATERIAL AND METHODS This was a cross-sectional study which included 90 known T2DM patients attending the Diabetic Out Patient Department of a tertiary care centre. This study was conducted after getting ethical committee clearance. Informed written consent was obtained from all the study participants after explaining the study to them. All participants were age and sex matched. INCLUSION CRITERIA: 1. Known cases of T2DM patients in the age group of 40-70 years. EXCLUSION CRITERIA: 1. Type 2 diabetics with UTI 2. Patients on treatment with ACE inhibitors or antihypertensive drugs 3. Patients with thyroid disorders and under thyroid medications 4. Patients on steroid therapy, nephrotoxic drugs 5. Patients with other renal disorders, cardiovascular disease, chronic liver disease and cancer. Detailed medical history and clinical examination was done for all the study participants. Height, weight, systolic blood pressure (SBP) and diastolic blood pressure (DBP) were recorded. Body mass index (BMI) was calculated for all the study participants. After an overnight fasting of 12-14 hours, about 5 ml of venous blood was drawn under aseptic precautions and collected into two test tubes. In test tube marked 1, 2 ml of blood was collected with sodium fluoride for measuring plasma glucose. In test tube marked 2, 3 ml of blood was collected without any anticoagulant and allowed to clot and serum separated was used for analyzing cystatin C. Cystatin C was estimated by Immunoturbidimetric assay. The reference values for cystatin C was determined to be 0.51 – 1.05 mg/L. Under aseptic precautions, spot urine sample was collected. Urine albumin and urine creatinine were estimated and albumin creatinine ratio was calculated in mg/g. Further these patients were categorized based on the urine albumin-creatinine ratio (UACR) into, Group A: Normoalbuminuria (UACR: < 30mg/g) Group B: Microalbuminuria (UACR: 30mg/g – 300mg/g) Group C: Macroalbuminuria (UACR: > 300mg/g) Cystatin c levels were assessed in all above 3 groups. Data was analyzed with appropriate statistical tests.
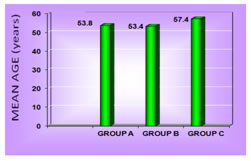
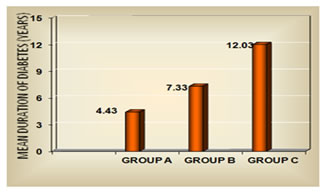
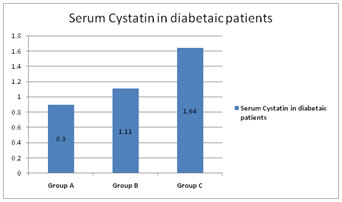
RESULTS Table1 shows that there is no significant association between gender and the normoalbuminuric, microalbuminuric and macroalbuminuric groups (p<0.05). The mean age was 53.8+9.1, 53.4+7.9 and 57.4+8.1 years for normoalbuminuric, microalbuminuric and macroalbuminuric groups respectively as shown in figure 1. The mean of BMI was 24.3+3.0, 24.3+2.4, 24.0+2.5 Kg/m2 for normoalbuminuric, microalbuminuric and macroalbuminuric groups respectively. No significant difference was found in the mean of BMI among the three groups as shown in table 2. The mean duration of diabetes was 4.43+2.43, 7.33+3.92 and 12.03+4.16 years for group A, B and C respectively. Figure 2 shows that there is statistically significant difference in the mean of duration among the three groups (p< 0.05). Mean of cystatin C was 0.94+0.23, 1.11+0.25 and 1.64+0.68 mg/L for the three groups respectively as shown in figure 3. Statistically significant difference was found in the mean of cystatin C among the diabetic groups (p<0.05). Table 3 shows that in group A, group B and group C, the normal cystatin C levels were 70%, 56.7% and 20% respectively. Table 1: Gender distribution among the diabetic groups
Figure 1: Age distribution of the diabetic groups
Table 2: Comparison of BMI among the diabetic groups
Figure 2: Duration of diabetes among the three groups
Figure 3: Cystatin C among the diabetic groups
Table 3: Distribution of patients according to diabetic group and serum cystatin C levels
DISCUSSION Diabetic nephropathy is a clinical hall mark of microangiopathy and is the most important single disorder that leads to renal failure in adults15. The earliest clinical evidence of nephropathy is the appearance of low but abnormal albumin levels in the urine and hence the early detection of nephropathy in diabetes mellitus patients has focused on the estimation of urinary albumin excretion rate. Type 2 diabetes usually develops after 40 years of age. In the present study, the age of diagnosis of diabetes was above 40 years for majority of the patients. The mean age was 53.8+9.1, 53.4+7.9 and 57.4+8.1 years for group A, B and C respectively. Hany S. Elbarbary et al16 and Assal et al17 had shown similar observations. In our study, mean BMI in the three groups were 24.29+3.0, 24.33+2.4, 23.96+2.5 Kg/m2 which was in the normal BMI range. This is in accordance with Assal et al17 and Jeon YK et al18 study. Even patients who are not obese as per the traditional weight criteria, may have an increased percentage of body fat distributed mostly in the abdominal region. These factors are thought to contribute to the higher insulin resistance and susceptibility to diabetes mellitus in South Asian Indians19. Duration of diabetes mellitus is very important factor in the development and progression of diabetic nephropathy which has been demonstrated in many studies. Longer the duration of DM, higher the frequency of developing diabetic nephropathy. Jiji Inassi et al20, confirmed and extended the frequent occurrence of microproteinuria with increasing duration of diabetes. In our study also, there was an increase in the duration of diabetes from group A to group C. The mean duration was 4.43+2.43, 7.33+3.92 and 12.03+4.16 years for group A, B and C respectively. Cystatin C is produced at a constant rate by nucleated cells in the body. It is released into bloodstream with a half-life of 2 hours and its concentration is almost totally dependent on GFR. In our study, mean of cystatin C significantly differed between the diabetic groups (figure 3). Hany S. Elbarbary16 stated that, the major advantage of cystatin C over creatinine is its ability to detect mild reduction in GFR to which creatinine is insensitive. Early detection of impaired renal function is very crucial to prevent the progression of renal disease and to improve the patient outcome. In our study, majority of the patients that is 43.3% in group B and 80% in group C had abnormal cystatin C level (table 3). In this way it is helpful in the early detection of kidney injury. In the present study, even in few normoalbuminuric patients, cystatin C levels were above normal value. This increment may be probably due to the tubular phase before glomerular manifestation21.
CONCLUSION Cystatin C may be considered as a useful, non-invasive marker for early detection of renal injury in T2DM patients.
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 Home
Home