Official Journals By StatPerson Publication
|
Table of Content - Volume 7 Issue 2 - August 2018
Ischemia modified albumin in early diabetic nephropathy
Mukherjee Brijesh1*, Sahoo Sibasish2
1Associate Professor, Department of Biochemistry, Hi-tech Medical College, Rourkela, Odisha, INDIA. 2Assistant Professor, Department of Biochemistry, SCB Medical College, Cuttack, Odisha, INDIA. Email: taararia@gmail.com
Abstract Background: Ischemia and hypoxia are observed in diabetic patients in addition to chronic hyperglycemia and intensified oxidative stress. It is reflected by increased markers levels of oxidative protein damage, especially in patients with vascular complications, as well as ischemia−modified albumin (IMA). Objective: To assess the plasma levels of IMA in T2DM patients with early diabetic nephropathy and their relationship with routine markers of kidney disease severity (urinary microalbumin, plasma creatinine concentration) Materials And Methods: Plasma IMA levels were manually determined in 100 patients and 50 healthy people by spectrophotometric Co(II)−albumin binding assay. Micoalbuminuria was detected using turbidimetric method and creatinine using Jaffe’s method in Cobas Autoanalyzer Results: The IMA levels in the diabetic patients were significantly higher than in the healthy controls and increased progressively with the degree of DN. The normoalbuminuria group 1 had the lowest IMA level, which significantly increased with development of microalbuminuria and as nephropathy progressed in group 2 and group 3. There were significant relationships between IMA and microalbuminuria (r = 0.87) Conclusion: This study indicates that the observed increased IMA in T2DM is connected with diabetic nephropathy. Measuring IMA plasma levels provided additional information about nephropathy development and it may be helpful in assessing the severity of oxidative stress, which causes kidney dysfunction at an early stage. Key Words: Diabetic nephropathy. Ischemia modified albumin.
Diabetic complications are associated with increased incidence, severity, and delayed recovery from the stress-induced ischemia participating in endothelial dysfunction, which may lead to the development of angiopathy, especially diabetic nephropathy (DN)1. The pathogenesis of DN is a complex process and it is not yet fully understood. The cause of increased urinary albumin excretion, which is considered to be an early sign of DN, can be attributed to a defect in the glomerular membrane filtration, resulting from renal endothelial damage due to hyperglycemia, oxidative stress, ischemia, and inflammation.2-5 However, several studies have reported development of DN in 29%–61% of type 2 diabetes patients having normal urine albumin levels, as microalbuminuria develops only when significant damage to the glomerular function has already occurred.6-8 This significant observation impedes the use of microalbuminuria as a good predictive marker9 and necessitates the need for a better biomarker with high sensitivity and specificity for early detection of DN.10 Free radical generation coupled with decreased antioxidant defence mechanism and associated ischemia in diabetes leads to an alteration in the tertiary structure of human serum albumin within minutes of arterial occlusion; hence, it is termed ischemia-modified albumin (IMA). As a result of increased generation of reactive oxygen species (ROS), structural modifications of the albumin molecule can occur. IMA is now being used as a sensitive biomarker for detecting cardiac ischemia as an indicator of oxidative stress.11 Recently, some studies were published regarding the role and diagnostic significance of IMA in noncardiac disorders. For the first time, Piwowar et al reported the association of IMA in diabetes and reported 75% increased value of IMA in diabetes compared with control after estimating it by manual spectroscopic method.12 Elevated IMA levels were also detected in diabetic ketoacidosis, pulmonary embolism, peripheral vascular disease, brain ischemia, infection, liver disease, trauma, some neoplasms, and complicated and uncomplicated pregnancies.13-16
MATERIAL AND METHODS The study was carried out in the Department of Biochemistry, after approval was obtained from the Institutional Ethics Committee on 8th October, 2016, from November 2016 to April 2018 under the guidance of Prof Dr P K Mishra and in collaboration with the Department of Medicine, HMCH, Rourkela. The selected diabetic patients visiting the Medicine OPD were evaluated for serum IMA, serum creatinine and urine microalbumin. The patients were divided into three groups as follows: Group I: comprised of 30 patients with duration of DM (diabetes mellitus) less than five years. Group II: comprised of 48 patients with DM duration between 5 to 10 years Group III: comprised of 22 patients with history of DM more than 10 years. The following inclusion and exclusion criteria were used to form the patient groups: Inclusion Criteria
Exclusion Criteria
Control group: 50 age and sex matched healthy individuals were taken as control. The following methods were employed for performing the various biochemical parameters:
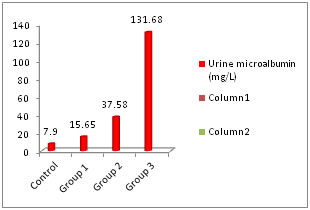
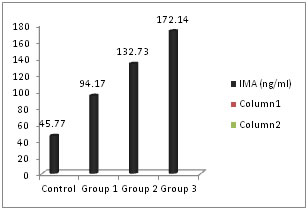
RESULTS The patients were divided in to three groups according to the duration of DM (<5 years, 5-10 years and >10 years respectively). The baseline characteristics of each group are presented as mean ± standard deviation (Table 1). Fasting glucose levels were higher in the study groups (group 3>group 2> group 1). However there was no significant increase in successive groups (p>0.05). Similarly HbA1c showed increase in successive groups without any significant increase (p>0.05). The average age of patients was highest in group 3 proving microvascular complication occur at a late age. Serum creatinine levels showed significant increase in group 3 when compared with control group (Table 2). This shows that creatinine levels increase when nephropathy progresses and cannot be an ideal marker for early nephropathy. Urine microalbumin increased significantly in group 2 and group 3 but was normal in group 1 (Figure 1). Serum IMA levels increased progressively as duration of diabetes increased (Figure 2). The levels were significantly higher in group 2 also compared with control group (Table 2). Table 1: Comparison of baseline characteristics among the groups
Table 2: Calculated p value by paired t-test after comparing each group with control.
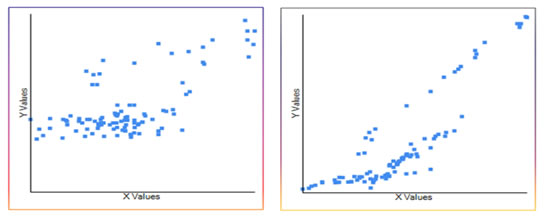
Figure 1: Urine microalbumin levels in different groups Both serum creatinine and urine microalbumin showed good correlation with serum IMA. The Pearson correlation coefficient (r) was 0.67 and 0.87 respectively when IMA was plotted in x-axis and creatinine and urine microalbumin was plotted in y-axis (Figure 3 and 4).
Figure 3: Pearson correlation coefficient (r=0.67). Serum IMA x-axis and serum creatinine y-axis; Figure 4: Pearson correlation coefficient (r=0.87). Serum IMA x-axis and urine microalbumin y-axis
DISCUSSIONS Diabetic patients are 17 times more prone to develop kidney disease than the normal population. The prevalence of DN is related to the prevalence of diabetes, which varies in different ethnic groups and geographical areas. The peak onset of DN is between 5-15 years after the onset of diabetes, as suggested in the study by Adler, et al.17 Patients who do not develop proteinuria after 20-25 years have only 1% risk of nephropathy, Gall, et al.18 A clinic – based study conducted at Dr Mohan’s Diabetes Specialties Centre (DMDSC) revealed that 9% of patients had proteinuria and 20-25% had microalbuminuria at any given time. In the Chennai Urban Population Study (CUPS), it was found that the prevalence of overt proteinuria was 2.2% and microalbuminuria was 26.3%. In the CURES population [19], the prevalence of overt proteinuria and microalbuminuria were, respectively, 2.2% and 26.9%. The prevalence rate is related to the duration of diabetes and increases in presence of risk factors like high HbA1c levels, hyperlipedemia and hypertension. These risk factors have been elaborated by studies done by Timothy et al.20. Taking all the above factors in to account, the study design of this study was framed accordingly. The study included diabetic patients having two risk factors (Serum Cholesterol >250 mg% and HbA1c >8%). This increased the chances of inclusion and evaluation of DN patients in the study, as evident from the various cited studies. This study results suggest that albumin modification occurs in the early stage of the disease provoked probably by hyperglycemia-induced stress and takes part in the pathogenesis of diabetic complications. IMA as an ischemic marker indicates underlying subclinical disease or vascular dysfunction in diabetic kidney, as suggested by Dash et al and Borderie et al21,22. Analyzing the relationships between IMA and parameters applied in the routine diagnosis of renal dysfunction, significant correlation was found with the urine microalbumin in all the three groups. With serum creatinine, IMA correlated only moderately and in group 3 (duration of diabetes >10 years), which proves that IMA is a helpful marker for detecting early renal dysfunction.
CONCLUSION The current study supports the essential relationship between the serum levels of IMA in type 2 diabetes patients and its utility in predicting vascular damage in DN. Increase in serum IMA level was more marked in diabetes without microalbuminuria and early DN cases compared with normal individuals, reinforcing the hypothesis that estimation of serum IMA may be used as a diagnostic marker to assess early detection and progression of nephropathy in diabetes. The clinical significance of IMA lies in its high negative predictive value, which may help in excluding the presence of early DN in a population. A cutoff value ≥89 ng/ml of serum IMA, as determined in this study, may help clinicians to diagnose early DN among normoalbuminuria cases or diabetes without microalbuminuria, which may help in the prognosis of the disease as well. Hence, along with the established markers such as serum creatinine and urine microalbumin, serum IMA estimation can be used as an auxiliary marker for early detection of DN.
REFERENCES
|
 Home
Home