Official Journals By StatPerson Publication
|
Table of Content - Volume 7 Issue 3 - September 2018
A study on genotypic frequency of 12-lipoxygenase polymorphisms and 12-HETE levels in type II diabetes
Suma M Nataraj1, Devananda Devegowda2*, Anisha Mathai3, Ankita Mysore Amarbabu3, Arpitha Bankenhalli Jayaram3, Pushkal Sinduvadi Ramesh4, Swetha Kempegowda5
1Professor and HOD, 2,5Assistant Professor, 3PG Student, 4PhD Scholar, 5Assistant Professor, Department of Biochemistry, JSS Medical College, JSS Academy of Higher Education and Research, Mysuru-15, Karnataka, INDIA. Email: devanandd@jssuni.edu.in
Abstract Background: Diabetes mellitus describes a group of metabolic diseases characterized by a progressive insulin secretory defect due to insulin resistance resulting in high glucose levels in the blood. Approximately 90% of all the cases of diabetes worldwide are type II Diabetes Mellitus where enough insulin is not produced for proper function. Growing body of evidence has suggested that beta cell inflammation and dysfunction play a key role in the pathogenesis of type II diabetes. Obesity and lack of exercise are some of the contributory factors for type II diabetes mellitus. A class of enzymes called lipoxygenases and derived oxidized lipids induces inflammation of beta cells and has been implicated in diabetes development. Studies show that the genetic deletion of 12-lipoxygenase (12-LOX) decreases the activity of 12-LOX and further decrease beta cell inflammation and dysfunction. Hence our main objective was to study the polymorphisms of 12-LOX gene in type II diabetes. Materials and Methods: Study participants included were in the age group of 30-70 years. 30 diagnosed patients of diabetes with the minimum history of 3 years were selected as cases. Equal number of age and sex matched healthy individuals were included in the control group. A detailed clinical history, family history and demographic details were collected from the subjects. Body Mass Index (BMI), HbA1c levels, Plasma 12-Hydroxyeicosatetraeinoic acid (12-HETE) levels and 12-LOX gene polymorphisms were assessed. Results and Conclusion: We observed significant difference in the routine parameters such as Fasting blood sugar (FBS), HbA1c and BMI whereas the 12-HETE levels did not show any significant difference between diabetic and non-diabetic subjects. Polymorphism study revealed varied genotype distribution of G>A and C>T gene clusters but again did not show any significance. Key Words: Gene polymorphism, 12-Lipoxygenase, Type II diabetes.
Diabetes has reached epidemic proportions in developing countries like India, showing an alarming rise in recent years. According to International Diabetes Federation (IDF), in India, 65 million people were suffering from diabetes in 2013 and the number is expected to increase to 109 million by 20351. Individuals with diabetes are at increased risk of developing cardiovascular disease (CVD) and globally, almost half of the 36 million deaths due to non-communicable diseases (NCDs) are caused by CVD. The incidence of CVD has gone up considerably in the age group between 25-69 years by 24.8%, which means loss of productive years and increase in economicburden2 Globally, type II diabetes accounts for more than 90% of the patients with diabetes and leads to various complications. Of the many complications, microvascular and macrovascular complications are the major ones causing profound psychological and physical distress to the patients as well as caretakers. This puts a huge burden on the health-care systems. Despite increasing knowledge regarding risk factors for type II diabetes, the incidence and prevalence of the disease continues to rise worldwide. Preventing or delaying complications can reduce morbidity and mortality which can be achieved through early detection via screening programmes and the availability of safe and effective therapies. Increased understanding of specific genetic phenotypes and genotypes might result in more specific and tailored management of patients with type 2 diabetes3 Though the reason for developing Type II diabetes is still not completely known, there are several other important risk factors such as obesity, poor diet, physical inactivity, increasing age, and family history.12-Lipoxygenase (12-LOX) is one among them, which belongs to a group of lipid peroxiding enzymes. There are considerable evidences to support that the role of 12-lipoxygenase enhances the development of diabetes and atherosclerosis4,5. In response to hyperglycaemia, the expression and the activity of 12-LOX are upregulated in a variety of metabolically active cell types [macrophages, adipose tissue hepatocytes, islet beta cells]. 12-LOX and its products appear to affect the function, survival of β cell islets and possibly cause differentiation6. The major product of 12-LOX is 12-HETE, which reduces glucose-stimulated insulin secretion in human islets at low concentrations (1nM) and induces islet death at higher concentrations (100nM). However, 15-HETE and the inactive form 12(R)-HETE have no effect on diabetes. Similar findings have also been observed in the mice islet7. Few investigators have studied the association between expression of 12-LOX and the levels of 12-HETE in the diabetic stage. But only a handful of studies are available that could prove this association. Given the importance of role of 12-LOX, it is essential we test the putative functional polymorphisms associated with type II diabetes mellitus. Therefore, recognizing this association might help in identifying subjects who are at high risk for developing diabetes or the ones at their early stages. Hence this study was taken up to assess the genotypic frequency of 12-LOX polymorphisms and 12-HETE levels in diabetic subjects and correlate the results with the healthy subjects.
MATERIALS AND METHODS This was a prospective, case-control study carried out in the Centre of Excellence in Molecular Biology and Regenerative Medicine laboratory of Department of Biochemistry, JSS Medical College and Hospital, Mysuru. The study was conducted after obtaining Institutional Ethical Clearance and signed informed consent from all the recruited subjects. Study Subjects: The sample size was calculated based on considering maximum mean difference of 12-Lipoxygenase between diabetic and non-diabetic subjects to be 50% with background standard deviation of 1, with 95% confidence level and 80% power of the study. Accordingly, 30 subjects were recruited for each group. Group I had newly diagnosed untreated type II diabetes mellitus subjects in the age group of 30-70 years. Group II had age and sex matched healthy individuals. Type II diabetes subjects were recruited as per the American Diabetic Association (ADA) criteria. Subjects who were on steroids, diuretics or with chronic illness including autoimmune disorders were excluded from the study. Demographic information related to the subjects such as age height, weight and family history was collected at the time of recruitment. Sample Collection: About 6mL of venous blood was collected under aseptic condition in a plain tube and EDTA tube for the estimation of blood glucose, HbA1c, lipid profile and 12-HETE levels along with DNA extraction respectively. The collected EDTA samples were subjected to centrifugation and plasma was separated. Once separated, the plasma samples were stored in cryo-vials at -800C freezer and thawed only at the time of 12-HETE estimation. Sample Analysis: Biochemical parameters such as fasting blood sugar and HbA1c of the subjects were analysed by glucose oxidase-peroxidase method using Toshiba Accute 40 FR and HPLC method using Bio-Rad D-10 system respectively. Estimation of human 12-Hydroxyeicosatetraenoic acid (12-HETE) by ELISA: To estimate the 12-HETE levels in the subjects, the plasma samples were subjected to ELISA using a standard kit from Cloud-Clone Corp (CCC, USA). This assay employed the competitive inhibition of enzyme immune assay technique. A monoclonal antibody specific to 12-HETE was pre-coated onto a microplate. A competitive inhibition was launched between biotin labelled 12-HETE analogues and unlabelled antigen (standards or samples) with the pre-coated antibody. After incubation the unbound conjugate was washed off. Next, avidin conjugated to Horseradish Peroxidase (HRP) was added to each microplate well and incubated. The amount of bound HRP conjugate was inversely proportional to the concentration of 12-HETE in the sample. After addition of the substrate solution, the intensity of color developed was inversely proportional to the concentration of 12-HETE in the sample. The yellow color developed was measured using a multimode plate reader (Perkin Elmer) at a wavelength of 450nm. DNA extraction: Blood collected in EDTA tubes were separated into plasma and blood cells. The blood cells were subjected to DNA isolation by non-enzymatic salting out method. The procedure involved lysis of RBCs, cell lysis using two grades of detergents, series of centrifugation steps and precipitation of DNA using isopropanol. The extracted DNA was quantified using a Nanodrop (DeNovix) and the quality was assessed by electrophoresing the DNA on 1% agarose gels stained with ethidium bromide and visualized using gel documentation system (Syngene). The integrity of the DNA was also assessed by amplification of house-keeping gene GAPDH by PCR. Genotyping of 12-LOX gene polymorphisms: An extensive search of NCBI databases was performed to find the single-nucleotide polymorphisms (SNPs) that have been informed within the 12-LOX gene. Of the 40 SNPs informed in the human ALOX12 gene, only six were located in the exons. Of these exonic SNPs, only four were non-synonymous. Among them two exonic SNPs with the cluster IDs rs1126667and rs2070589 corresponding to NCBI were considered for the study. A conventional PCR was performed to amplify and detect the presence of 12-LOX gene using the primers synthesized commercially for the respective cluster IDs. For the polymerase chain reaction, a brief master mix was prepared with 2.5units of Taq Polymerase (Himedia), 200nM of dNTPs, 10X buffer with 25mM MgCl2 (working 1X concentration), 0.4µM of each forward and reverse primers and 100ng of template DNA. The remaining of the reaction was made up to 25µL using PCR grade water. Amplification was carried out in Mastercycler Gradient (Eppendorf).
Table 1: Details of polymorphisms, gene cluster ID, primer sequences used in the study
Amplification was carried out with the above mentioned primers at 950C for 5mins followed by 30 cycles of 950C for 1min, 600C for 45sec, 720C for 1min and a final extension of 720C for 5mins. The amplified PCR products were electrophoresed on 2% agarose gels stained with ethidium bromide. The gels were run for 2 hours with 50 volts and the bands at expected product length was compared with the ladder and then considered whether positive or negative. Positively amplified PCR products were digested with the restriction enzyme Hin1I (for C>T) and Eco24I (for G>A) and electrophoretically resolved on 3.5% agarose gel and visualized under ultraviolet illumination. The TT genotype appears as a 663-bp band, while the digested pattern of CC genotype appears as 481- and 182-bp bands. The GG genotype appears as a 122-bp band, while the AA genotype appears as 244- and 127-bp bands. Statistical Analysis: All the data were put together in Graph Pad Prism 5.0 software and student t test was performed to assess the p value. P value less than 0.05 was considered to be significant. OBSERVATION AND RESULTS The results of the present study is based on the observation done in 30 type II diabetic and 30 age and sex matched healthy control subjects. We measured fasting blood glucose, HbA1C, BMI, 12-HETE levels in each participant and looked for statistical significance. Table 2: Demographic profiles of the study population
Table 3: Various parameters measured between type II diabetic and healthy subjects (The data is expressed in mean±SD, p value <0.05 was considered to be statistically significant)
Table 4: Genotype distribution frequency of 3957 G>A and 1559 C>T polymorphism in type II diabetic and healthy subjects
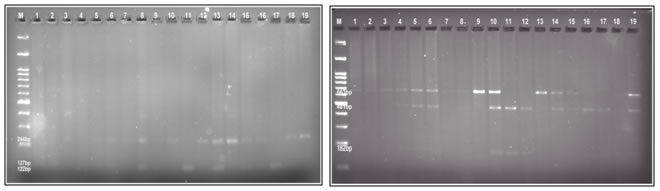
Figure 1 Figure 2 Figure 1: Representative gel image G>A amplified gene products digested with restriction enzyme Hin1I and electrophoretically resolved on 3% agarose gel (M- 100bp DNA marker). Figure 2: Representative gel image C>T amplified gene products digested with restriction enzyme BsaH1 and electrophoretically resolved on 3% agarose gel (M-100bp DNA marker). DISCUSSION Type II diabetes mellitus is increasingly common, primarily because of increase in the prevalence of sedentary lifestyle and obesity. Although the diabetes care is improving by many measures, complications are still common, and diabetes remains the leading cause for visual loss, amputation and end stage renal disease worldwide8. One of the main causes for macrovascular and microvascular complications associated with type II diabetes mellitus is oxidative stress9-11. The lipoxygenase (LOX) and cyclooxygenase (COX) generates Reactive oxygen species (ROS) indirectly by promoting the formation of inflammatory mediators that leads to obesity induced oxidative stress. The oxidative stress leads to β-cell dysfunction, impaired glucose tolerance and ultimately Type II DM12,13. In our study, we looked into the routine parameters such as BMI, fasting blood glucose and HbA1c levels in type II diabetic and non-diabetic subjects. It is a well-known fact that all these parameters are increased up to 10-folds and many of the investigators have shown the same14-16. In concordance with the previous findings, our study too showed a significant correlation in the FBS, HbA1c and BMI values when compared between the diabetic and non-diabetic groups. It should be noted that all these parameters could be controlled just by lifestyle modifications and only the complicated cases requires drug intervention. 12-HETE, which is a product of 12-LOX gene, is shown to induce oxidative stress and thereby put the pressure on degeneration of beta cells. Increased levels of 12-HETE is reported in many animal models as well as in human cohort studies5,17. Our data showed higher levels of 12-HETE in type II diabetic subjects when compared to the healthy controls. However, the levels were not of any significance which contradicted the existing data (refer table 3). We also assessed the genotypic frequency of two gene clusters of 12-LOX to identify the gene polymorphisms associated in the development of diabetes. Our data showed that G>A polymorphism genotypes were equally distributed in the diabetic subjects (33%), whereas in the non-diabetic subjects AA genotype was at 33%, heterozygous genotype GA was at 26% and none of the subjects were of GG genotype (refer table 4). Assessing the C>T polymorphism genotypes revealed that in the diabetic subjects the heterozygous CT and homozygous CC genotype was higher than in the non-diabetic subjects (53% vs 26% and 26% vs 13% respectively). But the TT genotype showed a reverse trend with the non-diabetic subjects showing more prevalence than the diabetic subjects (60% vs 20%). As per our knowledge this is the first study emulating from India studying the gene polymorphisms of 12-LOX in diabetic subjects. A large cohort study conducted in hypertensive subjects by Quintana et al demonstrated higher percentages of the above mentioned genotypes. They also showed a significant difference in the genotypic ratio between hypertensive and normotensive subjects18. On the other hand we also observed that there was no significant difference in the 12-LOX product, 12-HETE levels between the groups. Our data leads to a lot of questions. Earlier researchers have shown the role of 12-HETE and 12-LOX in inducing diabetes and also have demonstrated increased levels of the same. In contrast, our study did not find any significant correlation between the 12-LOX polymorphisms and type II diabetes. In the last few years, life style modification has been the main trend in the medically diagnosed diseased group and has actually lead to the decrease in the prevalence of many metabolic disorders. With the constant change in the gene signatures of a metabolic disease, eventually the dominant phenotype thrives and that affects the outcome of that particular gene function. Our small study population could also be the reason behind insignificant data, but nevertheless large multi-centric studies are further required to understand the association of 12-LOX gene polymorphisms among diabetic subjects in the Indian population.
CONCLUSION In our study, basic parameters like BMI, FBS and HbA1c levels showed significant correlation with the diabetes. Although 12-HETE levels and 12-LOX polymorphism genotypic ratio was higher in diabetic subjects than the non-diabetic ones, it was statistically not significant. Since other studies reveal that 12-LOX plays an important in beta cell destructions, future large scale studies may further throw light on the role of 12-LOX in diabetes and utilization of plasma 12-HETE as early markers for predicting the onset and progression of diabetes.
REFERENCES
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 Home
Home