Official Journals By StatPerson Publication
|
Table of Content - Volume 7 Issue 3 - September 2018
Regulatory role of HDL on systemic inflammatory response in adult bacterial sepsis
Naga Mrudula M1, Lingraj Patil2*
1Assistant Professor, Department of Biochemistry, Kanachur Institute of Medical Sciences, Manglore, Karnataka, INDIA. 2Tutor, Department of Biochemistry, GIMS, Gulbarga, Karnataka, INDIA. Email: mrudula1611964@gmail.com
Abstract Background: Inspite of the recent advances available in the intensive care units (ICU) there is still an increasing incidence of sepsis and sepsis related deaths. Recent studies suggest that high density lipoprotein (HDL) neutralizes and helps in clearance of lipopolysaccharide (LPS) or lipoteichoic acid (LTA) from circulation by the liver. Aims and Objectives: To conduct a prospective case control study to determine whether circulating HDL is a critical predictor of risk and severity of bacterial sepsis. Materials and Methods: During the study period 234 adult patients were clinically diagnosed to have sepsis. Out of these 35 patients were reported to have positive blood culture (confirmed sepsis group or Group 1). Thirty five age and sex matched patients were randomly selected from the suspected sepsis patients as Group 2. Thirty five normal healthy age and sex matched adults were taken as Group 3 (controls). Venous blood samples were collected from all these patients before the administration of antibiotics. The collected blood sample was used for complete blood count, HDL and CRP estimations. The gold standard for the diagnosis of sepsis was positive blood culture. Results and Observation: The CRP levels were significantly increased and HDL levels were significantly low in confirmed sepsis (p<0.0001) and suspected sepsis (p<0.0001) when compared to that of control subjects. HDL levels <32.25mg/dl showed 91.18% sensitivity and 75.0% specificity to differentiate between confirmed sepsis and healthy subjects. There was a significant negative correlation between HDL and CRP. The overall correlation coefficient r is -0.54 and P value is <0.0001. Conclusion: From this study, it is clear that HDL levels are significantly decreased in response to bacterial infection or inflammation. The decrease in HDL is well correlated with increased levels of CRP. HDL levels in bacterial sepsis may have a value in identifying patients with infection. Key Word: bacterial sepsis. INTRODUCTION Sepsis is defined as the presence (probable or documented) of infection together with systemic manifestations1. In a multicentric observational study conducted in India from June 2006 to June 2009 it was reported that the incidence of severe sepsis was 16.45% of all the admissions in the intensive therapy units. Hospital mortality and 28-day mortality of severe sepsis were 65.2% and 64.6%, respectively2. Inspite of the recent advances available in the intensive care units (ICU) there is still an increasing incidence of sepsis and sepsis related deaths. Despite conducting more than 100 randomized clinical trials directed towards modulating immune response to infection, none of them have resulted in new treatments for sepsis. This warrants a multidisciplinary new line of biochemical epidemiological studies and mechanistic understanding of bacterial sepsis to improve diagnostic tools and develop new therapy. In sepsis, the pathogenic lipids present on bacterial cell wall, primarily lipopolysaccharide (LPS) in Gram negative and lipoteichoic acid (LTA) in Gram positive bacteria binds to Toll-like receptors (TLR) present on immune effector cells and trigger uncontrolled inflammatory responses. The host factors that can directly inactivate these pathogenic lipids or blunt TLR downstream signaling are critical modifiers of sepsis pathogenesis3. High density lipoproteins (HDL) besides transporting cholesterol from peripheral tissues to liver for excretion is a major lipoprotein that sequesters, neutralizes and helps in clearance of LPS or LTA from circulation by the liver (4). Furthermore, growing evidences suggest that HDL has antioxidant, anti-inflammatory, anti-microbial and immunoregulatory activity. These protective roles are attributed to HDL proteome that includes apolipoprotein A1 (ApoA1), Lecithin-cholesterol transferase, paraoxonase 1, and platelet activating factor (PAF) acetyl hydrolase5. HDL sequesters acute phase proteins such as serum amyloid A, C-reactive proteins that are elevated during infection and are critical late-mediators of sepsis6. It also inhibits bacterial growth and prevents dissemination and therefore, deficiency of HDL compromises host defenses against bacteria. Low levels of HDL inversely correlate with exaggerated systemic inflammation and severity of sepsis in adult patients. Low HDL levels are also associated with increased mortality in ICU patients7. Depletion of HDL by knocking out ApoA1gene exacerbated whereas administration of ApoA1 mimetic inhibited systemic inflammation and mortality in mouse models of sepsis8. HDL attenuates NF-KB-TLR4 inflammatory signaling via ATF4; protects from vascular endothelial cell activation and injury by regulating iNOS9. Administration of reconstituted or synthetic HDL mitigated systemic inflammation in human subjects injected with LPS (human endotoxemia model)10. In view of the above data, we hypothesise that low HDL level is an important risk factor for bacterial sepsis and are associated with exaggerated systemic inflammatory response.
MATERIALS AND METHODS Source of data Patients with suspected sepsis on clinical grounds, admitted to the ICU of a tertiary care hospital, who met all inclusion criteria, were enrolled in the study. Ethical committee approval was taken before the commencement of the study. Written consent was obtained from the patient’s attender to use the blood sample for research purpose. Inclusion criteria: Patients with age greater than 18 years and satisfying the criteria for severe sepsis according to International guidelines for management of severe sepsis and septic shock: 2012(10), and who had not received antibiotics were included in the study as cases. Normal healthy age and sex matched adults were included as controls. Exclusion criteria: Patients on hypocholesterolemic drugs and those who have already received antibiotics before admission were excluded from the study. Also patients with known chronic inflammatory conditions like Human immunodeficiency virus (HIV) disease, SLE (Systemic lupus erythematosus) and RA (Rheumatoid arthritis) were excluded. Sample size: During the study period 234 adult patients were clinically diagnosed to have sepsis. Out of these 35 patients were reported to have positive blood culture. This group was taken as confirmed sepsis group (Group 1). Thirty five age and sex matched patients were randomly selected from the suspected sepsis patients as Group 2. Thirty five normal healthy age and sex matched adults were taken as Group 3 (controls). Venous blood samples were collected from all these patients before the administration of antibiotics. The collected blood sample was used for complete blood count, HDL and CRP estimations. The gold standard for the diagnosis of sepsis was positive blood culture. The blood culture bottle was loaded into the Bactec 9240 automated blood culture instrument. For bottles that flagged positive by the Bactec instrument had fluid withdrawn for gram stain and subcultured on the appropriate agar-based culture plates like blood agar, MacConkey agar and Salmonella Shigella agar. Pure colonies were further sub-cultured on Mueller-Hinton agar for antibiogram study using Kirby-Bauer Disk diffusion method and results were analyzed by an automated identification system along with the appropriate biochemical reactions for phenotypic identification. HDL and CRP were estimated using fully automated clinical chemistry analyzer RandoxImola from Randox laboratories Ltd. HDL was estimated by direct HDL-cholesterol method. The linearity of the method is upto144mg/dl and the minimum detectable concentration with an acceptable level of precision, is 7.30 mg/dl. CRP was estimated by immunoturbidimetric assay. The linearity of this Assay is upto220 mg/L and the minimum detectable concentration with an acceptable level of precision, is 2.88 mg/L. Statistical analysis Arithmetic mean and standard deviation (SD) were estimated to assess the level of various parameters in the study. Differences in the levels of CRP and HDL between the defined groups were assessed using the Students‘t’ test (if the comparison was between two groups) or repeated measure forward ANOVA (if the comparison was between more than 2 groups). Sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and positive or negative likelihood ratios (+LR) for the study parameters in predicting inflammation or infection was calculated. Also, receiver operative characteristics (ROC) curves were constructed for each of the predictive variables and the areas under the ROC curves (AUC) were compared. The analyses were facilitated with the use of the MedCalc 16.8 software packages. Differences were considered significant if the P value was <0.05. RESULTS The clinical characteristics of the subjects included in the study are shown in table 1. A total of 105 subjects were included in the study with 35 subjects in each group. There were 19 males and 16 females in the control group, 17 males and 18 females in both suspected sepsis group and confirmed sepsis group. Mean age of the control group was 49.7±17.9, suspected sepsis group was 50.1±17.7 and confirmed sepsis group was 51.6±17.2. The mean and standard deviation of CRP and HDL in the confirmed sepsis, suspected sepsis and control group is also shown in table 1.
Table 1: Clinical characteristics of the study population along with the study parameters
The average HDL and CRP values for the control group were 40.19±11.8 and 3.49±7.6 respectively. In the suspected group these values were 24.57±14.03 and 118.72±86.6, whereas in the confirmed group they were found to be 19.21±12.67 and 144.7±81.6 respectively. The CRP levels were significantly increased in confirmed sepsis (p<0.0001) and suspected sepsis (p<0.0001) when compared to that of control subjects. The levels of HDL were significantly low in confirmed sepsis (p<0.0001) and suspected sepsis (p<0.0001) when compared to that of control subjects. However, the levels did not significantly differ between the suspected sepsis and confirmed sepsis group (CRP p=0.20, HDL p=0.10). There was a significant difference in the number of days of stay in the hospital between the suspected sepsis and confirmed sepsis group (p=0.0059). The list of organisms isolated from the confirmed cases of sepsis is shown in table 2. E.coli was the most common organism isolated followed by Staphylococcus species and Klebsiella pneumoniae.
Table 2: Pathogens isolated in blood cultures from 35 patients with bacteremia
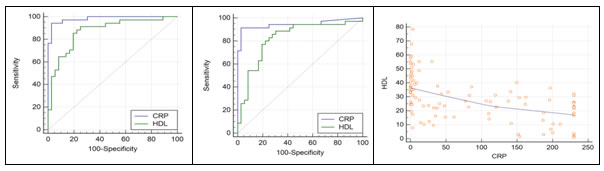
The sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), positive or negative likelyhood ratio (LR) and area under the receiver operative curves (ROC) (AUC) of CRP and HDL to differentiate between control and confirmed sepsis as well as to differentiate between control and suspected sepsis is shown in Table 3 and Figure 1and 2. TABLE 3: Predictive ability of the study parameters CRP and HDL to differentiate between the Control/Confirmed and Control/Suspected
Figure 1 Figure 2 Figure 3 Figure 1: Comparison of the receiver operative curves of CRP and HDL between controls and confirmed sepsis; Figure 2: Comparison of the receiver operative curves of CRP and HDL between controls and suspected sepsis; Figure 3: Correlation Graph HDL versus CRP CRP level >12.03mg/L showed 94.12% sensitivity and 97.22% specificity to differentiate between confirmed sepsis and healthy subjects and 91.43% sensitivity and 97.22% specificity to differentiate between suspected sepsis and healthy subjects. HDL levels <32.25mg/dl showed 91.18% sensitivityand 75.0% specificity to differentiate between confirmed sepsis and healthy subjects. HDL levels <33.77md/dl showed 88.57% sensitivity and 69.44% specificity to differentiate between suspected sepsis and healthy subjects. There was a significant negative correlation between HDL and CRP. The overall correlation coefficient r is -0.54 and P value is <0.0001
DISCUSSION In this study, we report that HDL levels were markedly low and CRP levels are high during sepsis. During sepsis, the considerable decrease in HDL levels relative to normal conditions remains unexplained. Several hypotheses are potential, including an acute over-consumption of HDL particles, a decrease in liver HDL synthesis (especially in cases of hepatic failure) or an increased clearance following an upregulation of scavenger receptors such as SRB-111. In the context of sepsis, HDL particles may also easily be redistributed from the intravascular to the extravascular compartment12,13. One of the major actions of HDL particles during sepsis is the clearance of bacterial components, such as lipopolysaccharides (LPS)14. After binding to the bacterial components, the clearance of HDL particles may also be increased in inflammatory conditions14,15. Interestingly, in the population of septic patients, HDL concentration was higher in patients with gram-positive versus gram-negative bacteria, suggesting that the clearance of HDL-LPS particles may be enhanced16. In this pathology, cell injury initiates the secretion of different mediators such as damage-associated molecular patterns (DAMPs), which are recognized by the host, leading to a pro-inflammatory response17,18. Also, similar leukocyte genomic responses to inflammation may be involved in sepsis patients19. The marked release of bacterial endotoxins may lead to an increased consumption of HDL particles. The potential protective role of HDLs has during sepsis. On one hand, Chien et al. have shown that HDL levels in non-survivors were significantly lower than those of survivors from day 1 to day 4 and that HDL-C concentration at day 1 could predict the overall 30-day mortality rate. In their study, the cut off value of 0.52 mmol/l had a sensitivity of 92% and a specificity of 80% for predicting the overall 30-day mortality rate [07]. Barlage et al., in an observational study in ICU, also underlined the relationship between apo-A1 levels and 30-day mortality20. On the other hand, Lee et al. reported no significant HDL differences between survivors and non-survivors in 117 septic patients and only Triglyceride levels were associated with mortality21. Cirstea et al. have reported that low HDL levels at the time of admission for suspected sepsis were strongly and independently prognostic of subsequent multiple organ dysfunction22. Our study correlates, the above studies, clearly that HDL levels significantly decrease in response to bacterial infections.
CONCLUSION From this study, it is clear that HDL levels are significantly decreased in response to bacterial infection or inflammation. The decrease in HDL is well correlated with increased levels of CRP. HDL along with CRP can be used as an important diagnostic indicator in bacterial sepsis. HDL levels <32.25mg/dl showed 91.18% sensitivity and 75.0% specificity to differentiate between confirmed sepsis and healthy subjects. HDL levels <33.77md/dl showed 88.57% sensitivity and 69.44% specificity to differentiate between suspected sepsis and healthy subjects. We further plan mechanistic studies to gain in-depth insight into HDL controlled molecular pathways in leukocytes that regulate the course of sepsis. HDL levels in bacterial sepsis may have a value in identifying patients with infection. Our findings can easily be translatable into clinical practice for risk assessment, diagnosis and management of bacterial sepsis.
REFERENCES
|
 Home
Home