|
Table of Content - Volume 21 Issue 3 - March 2022
Study of hepatic dysfunction in dengue fever
Pratap P Budhya1, Akshayakumar Arjunagi2, Shubham Malawadi3, Vilas Honnakatti4*
1,4Assistant Professor, 2Senior Resident, Department of General Medicine, Belagavi Institute of Medical Sciences and Hospital, Belgaum, Karnataka, INDIA. 3Post Graduate, Department of ENT, Shivamoga Institute of Medical Sciences and Hospital, Shimoga, Karnataka, INDIA. Email: vilasdoc84@gmail.com
Abstract Background: Dengue Fever has been recognized as one of the fastest spreading arboviral infection worldwide. Its manifestations can be benign in its classical form and serious in Dengue Haemorrhagic Fever (DHF) and Dengue Shock Syndrome (DSS), also may involve multi-organ system. The objective of this study was to determine the incidence of Hepatic dysfunction in patients with Dengue Fever and assess the prognostic implications of hepatic dysfunction. Materials And Methods: This study is a hospital based prospective study done over a period of one and half years on 100 subjects of Dengue Fever, selected randomly and included in the study based on inclusion and exclusion criteria. A detailed history was taken, clinical examination and investigations were done and details entered in a structured proforma. The data collected was transferred into a Master Chart which was subjected to statistical analysis. Patients were categorized into mild, moderate and severe Hepatitis group based on aminotransferase levels. Results: Total of 100 Dengue cases were included in the study out of which 52 were males and 48 were females. Mean age of the patients was 41.7 years. Based on WHO criteria, 5 patients had DHF, 2 were diagnosed as DSS and remainder 93 had classical dengue fever. Out of 100 patients, Hepatic dysfunction was seen in 85% of patients with aminotransferase levels being elevated, 40% had mild hepatitis, 34% had moderate and 11% had severe hepatitis. There was no statistically significant correlation between Length of Hospital Stay and severity of Hepatitis. There were 3 deaths due to Dengue during the course of study with 2 patients having moderate hepatitis and 1 having severe hepatitis and there was no statistical significance between mortality and severity of hepatitis. Conclusions: In this study, we conclude that Hepatic dysfunction is seen in most of the patients with dengue fever and should be considered as one of the differentials for acute febrile illness with hepatitis. However, the prognostic significance of liver function tests in Dengue fever needs to be determined by larger studies. Key Words: DHF; Dengue Hemorrhagic Fever, DSS; Dengue Shock Syndrome, Hepatic Dysfunction, Length of Stay, Thrombocytopenia.
INTRODUCTION Dengue Fever is an acute febrile illness of viral aetiology, evolution of which is benign in its classical form and serious in Dengue Haemorrhagic Fever (DHF) and Dengue Shock Syndrome (DSS). There are four distinct serotypes of dengue viruses with Aedes mosquito as their principal vector and all cause a spectrum of similar clinical syndrome. Around 2.5 billion people i.e. 2/5th of world’s population in tropical and subtropical countries are at risk of the disease.1 Approximately 90% of them are children aged less than 5years and have a mortality rate of 2.5%. The countries of South East Asia region are divided into 3 categories A, B, C and India comes under Category A.1 This shows that Dengue is a major health problem, leading cause of hospitalization and death, hyper endemic with all 4 serotypes circulating in urban areas and spreading to rural areas. AIMS AND OBJECTIVES: To study the impact of Dengue fever on liver function. To determine whether evaluation of liver function parameters can be of prognostic value.
MATERIALS AND METHODS Study Design: Clinical, prospective and observational study. Study Duration: January 2015 to June 2016. Study Site: Justice K S Hegde Charitable Hospital, A unit of NITTE University. Sample size:100 cases of Dengue Fever. Sample selection- Random. Inclusion Criteria: WHO Criteria of Dengue fever2 –Acute febrile illness plus 2 or more of the following: Headache, retro-orbital pain, myalgia, arthralgia, nausea/vomiting, skin rash and supportive serology in the form of IgG/ IgM antibody specific to dengue virus or detection of dengue virus antigen (Dengue card test positive). WHO criteria for Dengue Hemorrhagic fever: Fever of 2-7 days. Bleeding manifestations indicated by positive tourniquet test /petechiae, ecchymosis, purpura/ bleeding per mucosa/hematemesis, malena. Platelet count <1,00,000/mm3. Plasma leakage evidenced by Rise in PCV >20%, fall in PCV by 20% after IV Fluids, Pleural effusion, ascites, hypoalbuminemia. WHO criteria for DSS: DHF + weak pulse, hypotension, narrow pulse pressure and cold dry skin, restlessness. Exclusion Criteria: Alcoholic liver disease Other causes of acute febrile illness with deranged LFT like Leptospirosis, Malaria. Drug induced Hepato-toxicity. METHODOLOGY After obtaining informed consent, 100 subjects were selected randomly and included in the study based on inclusion and exclusion criteria. A detailed history was taken, clinical examination and investigations were done and details entered in a structured proforma. The data collected was transferred into a Master Chart which was subjected to statistical analysis. Hepatic dysfunction, for assessing the prognosis was divided into mild, moderate and severe as per the aminotransferase levels.3 SGOT or SGPT between 40-100 U/l = mild dysfunction SGOT or SGPT between100-300 U/l= moderate dysfunction SGOT or SGPT >300 U/l = severe dysfunction SELECTION OF CASES Statistical Analysis: The data collected was subjected to Descriptive analysis and Chi Square test. Results were compared using One way ANOVA test. Post hoc tukey test was used for sub group analysis. Linear regression analysis was done for analysis of thrombocytopenia and hepatic dysfunction in predicting length of stay. P value of < 0.05 was taken as statistically significant.
RESULTS There were 52 males and 48 females in the study
Table 1: Showing baseline characteristics and LFT in the study
Table 2: Showing comparison of length of stay with hepatic dysfunction LOS= length of stay
Table demonstrates that mean length of stay in patients with no hepatic dysfunction was 5.07 days whereas in those with severe hepatitis it was 5.45 days. P value being 0.12 (not significant)
Table 3: Showing subgroup analysis of length of stay between groups of hepatic dysfunction
Post hoc Tukey test, a statistical test for subgroup analysis applied to compare length of hospital stay with degree of hepatitis, shows that length of stay does not vary significantly when hepatic dysfunction group was sub analysed.
Table 4: Comparing severity of hepatic dysfunction v/s outcome
TABLE 5: CHI-SQUARE TEST FOR DEATH IN HEPATIC DYSFUNCTION
The P value of Pearson Chi-Square is 0.246, indicating that there is no statistical significance between death and severity of hepatic dysfunction
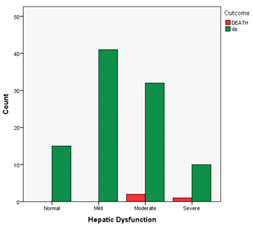
Figure 1: Bar diagram showing number of deaths v/s severity of hepatic dysfunction The bar diagram depicts that there were total 3 deaths, of which 2 were in moderate hepatitis group and 1 in severe hepatitis group
DISCUSSION Dengue fever is one of the most rampant arboviral infections all over the world. It is known to involve multi organ system and involvement of liver is one among them. During the study period of 18 months, 227 cases of dengue fever were admitted in our hospital of which 12 were of paediatric age group, 8 patients had concurrent CLD and 3 patients had drug induced hepatitis (on Anti Tubercular drugs). A total of 100 dengue cases were randomly included in the study as reports of other patients were not generated in our hospital. Age and Gender Distribution: In our study there was almost equal distribution of cases in both the sexes. There were 52 males and 48 females. Mean age of the affected patients was 41.7 years suggesting that the working age group is mainly affected, as per this study, probably because as they are exposed to more mosquito bites specifically in fields and outdoors. The mean age in this study was much higher when compared to the study done by Vaibhav Shukla.4 LIVER FUNCTION TEST: In our study, 85% (85/100) had hepatic dysfunction based on liver function abnormality. 15% (15/100) had no elevation of SGOT or SGPT, a finding similar to study done by Ali K Ageep.5 The only pattern of abnormal LFT seen was of Acute hepatocellular necrosis type and none had haemolytic or cholestasis variety of liver function abnormality. Among those who had abnormal LFTs i.e. out of 85, 40 had mild hepatitis, 34 had moderate and 11 had severe hepatitis with enzyme levels >300 U/l. Highest recorded SGOT was 1205 U/l with mean of 158.6 (std. deviation 209.01) U/l and SGPT was 796U/l with mean of 99.07 (std. deviation 120) U/l. Ratio of means of SGOT and SGPT is 1.6:1 showing that there was no preferential increase in liver enzymes in our study unlike as seen in other studies4, 6 where the ratio of SGOT:SGPT was >2:1. HEPATIC DYSFUNCTION AND PROGNOSIS: We determined the prognostic implication of hepatic dysfunction by comparing it against platelet counts and short-term outcome like Length of stay (LOS) and In Hospital Mortality. HEPATITIS AND LENGTH OF STAY: In our study, 15 had no hepatitis and in that group the mean length of hospital stay was 5.07 (1.62) days. In mild hepatic dysfunction group it was 4.17 (1.85) days, in moderate hepatitis group it was 5.35 (3.0) days and 5.45 (2.52) days in those who had severe hepatitis. This shows that when compared to no hepatitis v/s severe hepatitis the mean LOS prolonged by only 0.38 days. This difference is statistically not significant with a test value of 1.992 and p value of 0.12. Post hoc Tukey test, a statistical test for subgroup analysis, shows that the difference in LOS between no hepatic dysfunction and Mild hepatitis is NOT statistically significant with a mean difference of -0.896 and p value of 0.579. The difference in LOS between no hepatitis and Moderate hepatitis group is NOT statistically significant with a mean difference of -0.286 and p value of 0.979. The difference in LOS between normal LFT and severe hepatitis is NOT statistically significant with a mean difference of -0.388 and p value of 0.975. The difference in LOS between Mild and Moderate hepatitis is NOT statistically significant with a mean difference of -1.182 and p value of 0.132. The difference in LOS between Mild and Severe hepatitis is NOT statistically significant with a mean difference of -1.284 and p value of 0.368. The difference in LOS between moderate and severe hepatitis is NOT statistically significant with a mean difference of -0.102 and p value of 0.999. However in a similar study conducted in the neighbouring country of Pakistan, there was significant prolongation in hospital stay in patients with severe hepatitis as compared with mild to moderate hepatitis7. HEPATIC DYSFUNCTION AND IN HOSPITAL DEATH: We observed 3 (3%) deaths in this study and remaining 97 got discharged. There was 2 deaths observed in moderate hepatic dysfunction group and 1 death in severe hepatitis. There was no mortality in mild hepatitis group. As it would be expected to have a higher mortality in patients with severe hepatitis as per the previous similar study3 and less in mild to moderate, it was not the trend in our study. When Chi-Square test was applied for death in hepatic dysfunction, the p value of Pearson chi-square was 0.246 indicating that there is no statistical significance between death and severity of hepatitis. OTHER OBSERVATIONS: In our study, clinical evidence of bleeding was seen in 6patients. In those patients, one had INR of 1.7, indicates that prolongation of INR may not have been the cause of bleeding. The platelet count in all of them was below 37000cells/mm3. Thus bleeding in them was probably due to thrombocytopenia and Prothrombin time did not correlate well in our study, a finding observed in the other study as well.5 As mentioned earlier there were 3 deaths in the study period, 2 males and 1 female. None of them had hepatomegaly and only 1 had severe hepatitis. One patient in the moderate hepatitis group had total bilirubin of 2.9mg/dl and was clinically icteric. He presented in shock but had no evidence of bleeding manifestations.
LIMITATION OF THE STUDY Short duration of study period. Small study population. Only blood reports at the time of admission was taken for study. All the patients did not present to the hospital on same day of their illnesses and thereby LFTs do not represent any particular day of illness and the rise and fall of liver enzymes with respect to the duration of illness could not be assessed. Since there were only 3 deaths, spectrum of liver injury inpatients who died cannot be extrapolated to general population.
CONCLUSION In this study, we conclude that Hepatic dysfunction is seen in most of the patients with dengue fever and should be considered as one of the differentials for acute febrile illness with hepatitis. However, the prognostic significance of liver function tests in Dengue fever needs to be determined by larger studies.
REFERENCES
Policy for Articles with Open Access
|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 Home
Home