|
Table of Content Volume 14 Issue 1 - April 2020
Prospective study of Apremilast in cases of chronic plaque psoriasis
Alok Kumar Agarwal1*, Padma2, Shahana3, G Narasimha Rao Netha4
1PG, 2,3Assistant Professor, 4Professor & HOD, Department of DVL, Gandhi Medical College, Secunderabad, Telangana, INDIA. Email: reachdoctor@gmail.com
Abstract Background: Psoriasis is a chronic inflammatory skin disease, which requires long-term, safe and effective treatment. Apremilast, an oral PDE4 inhibitor, works intracellularly to regulate inflammatory mediators. It was approved by the FDA in 2014 and by the EC in 2015 for the treatment of Chronic Plaque Psoriasis. Aims and Objectives: To evaluate therapeutic responses of Apremilast in cases of Chronic Plaque Psoriasis (CPP). Methods: We included 21 patients affected by chronic plaque psoriasis aged >18 years and were evaluated every 4 weeks, and we documented: age, family history of psoriasis, joint involvement, previous treatments, psoriasis area severity index (PASI) scores, and the onset and duration of adverse events(AE). Efficacy was analysed by PASI50, PASI75 and PASI90, reflecting the improvement of skin lesions compared to the PASI-baseline. Results: Twenty-one patients with chronic plaque psoriasis were included in the study. Twelve patients (57.14%) reached PASI 75 and three patients (14.28%) had reached PASI 50.A statistically significant improvement of PASI scores was observed relative to pretreatment measurements. Five patients (23.8%) reported at least one AE, most frequently diarrhoea (n = 3, 14.28%), headache (n = 2, 9.52%) and joint pain (n = 2, 9.52%). Conclusion: Apremilast is a safe and valuable therapeutic modality for patients with chronic plaque psoriasis.Up to 70% of the patients will reach PASI50 or better. These results, from a prospective study, confirm the efficacy and safety of apremilast. Key Word: Apremilast.
INTRODUCTION Psoriasis is a chronic, recalcitrant inflammatory skin disease affecting 2 – 3% of the world population.1,2 Chronic plaque psoriasis is the most prevalent form and accounts for > 80% of patients with psoriasis. Dendritic cells (DC) and T cells mediate the inflammation in psoriatic skin. Myeloid DC secrete interleukin (IL)-12 and IL-23 that activate T-helper-cells (Th1, Th17, Th22), which produce IL-17, tumour-necrosis-factor (TNF), interferon (IFN) ƴ and IL-22. These cytokines stimulate keratinocytes to proliferate so rapidly that proper differentiation to mature cornified cells is not achieved.3 – 8Patients with mild disease can often be treated efficiently with topical preparations; however, those with moderate to severe disease require systemic therapies including photo (chemo) therapy with UVA and UVB, retinoids, methotrexate, cyclosporine, or newer biologic agents (biologics) and their biosimilars.9 – 12.Such bio-logics have changed the therapeutic landscape, and most patients benefit greatly from these new modalities.13.Still, not all patients respond to such systemic antipsoriasis therapies. Percentages of patients reaching PASI75 or better (response rates) vary among different agents: methotrexate 60% after16 weeks, cyclosporine 50% after 8 weeks, retinoids 25 – 50 (variable according to dose), TNF-Alpha inhibitors 30 – 80% (variable according to agent used) after 16 weeks, ustekinumab 60 – 70% after 12 weeks.11,14,15Apremilast is an oral phosphodiesterase-4 (PDE4) inhibitor which has been approved by the US Food and Drug Administration (FDA) in 2014 and by the European Medicines Agency (EMA) in 2015.16The drug is approved for the treatment of chronic plaque psoriasis in adults who failed to respond to or have contraindications to other systemic therapies. Its use does not need laboratory monitoring, and its oral route of administration gives it an advantage compared to biologics and other systemic antipsoriasis drugs.16 The drug regulates immune responses associated with psoriasis by inhibiting PDE4, which is highly expressed in DC, monocytes, neutrophils and keratinocytes, where it degrades cyclic adeno-sine 30,50-monophosphate (cAMP).17 This inhibition results in higher intracellular cAMP levels and leads to a correction of the cytokine imbalance, by increasing anti-inflammatory and decreasing pro-inflammatory cytokine production. This is evidenced by the reduction in important pro-inflammatory cytokines TNF- α , IL-6, IL-17 and IL23 in patient plasma and IL-17and IL-23 in psoriatic skin.18 – 20 Given its mechanism of action ,apremilast acts at an earlier step in the inflammatory cascade, which gives us further rationale for the initiation of apremilast before switching patients with psoriasis to biologics. Randomized trials have documented the efficacy and good safety profiles of apremilast; yet, prospective studies on real-world patients are scarce.19,21 – 24In this study, we prospectively recorded various clinical data of psoriasis patients treated with apremilast. We describe efficacy and adverse events (AE) as an important and crucial factor reflecting a drug’s long-term usage in real-life settings.25
MATERIALS AND METHODS Patients and data collection The study was conducted at Department of DVL, Gandhi Medical College, Telangana. We included 21 patients affected by chronic plaque psoriasis aged >18 yrs and willing to participate in study. Apremilast was used following its prescription recommendations (start 10 mg/day, stepwise increase to 30 mg twice/day). All patients were recommended to use additional topical treatments. None of the patients received additional systemic antipsoriatic therapy. Patients did not participate in any other clinical trials and signed consent forms for the anonymized use of their data. The ethical committee of GMC approved the study. All patients were evaluated at predefined time points (week 0, 4, 8,12, 16, 20, 32 and 40). At each visit, the following data were noted - family history of psoriasis, joint involvement, previous psoriasis treatments, psoriasis area severity index (PASI) scores, and the onset and duration of adverse events (AE). Data analysis Treatment efficacy was evaluated by PASI50, PASI75 andPASI90, reflecting the improvement of skin lesions compared to PASI-baseline (PASI calculated at the beginning of treatment).26,27 Psoriasis severity was classified based on PASI as mild (PASI < 10) and moderate – severe (PASI ≥ 10).Cohort PASI scores were calculated for weeks 4, 8, 12, 16, 20, 32and 40. Descriptive statistics were used to express patient demographics and AE distribution. The variable selection was based on published literature.
RESULTS Twenty one patients with chronic plaque psoriasis were included in the study(Table 1). The median age at the time of the first apremilast dose was 40 years (range 20-65), and 13 patients (62%) were males.9 patients (42.8%)) had a positive family history of psoriasis. Most of the patients (n=10,47.6%) received at least one systemic psoriasis treatment prior to apremilast. No patient had a drug washout period prior to switching to apremilast. At the beginning of apremilast treatment, the mean PASI in our study cohort was 10.7 (range 3 – 22.7).
. Table 1: Baseline demographics and clinical characteristics of 21 psoriasis patients treated with apremilast
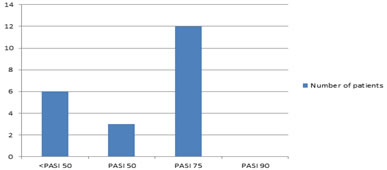
To analyse treatment efficacy in our cohort, we were able to include 21 patients (Fig. 1). Using the measure ‘best PASI reached’, 3 patients (14.28%) had at least a PASI-50 and 12 patients (57.14%) a PASI-75. None of the patients reached PASI-90. The best treatment response was achieved between weeks 12 and 16.
Figure 1: Bar graph depicting the best PASI response
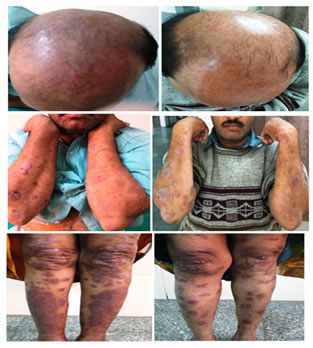
CLINICAL PHOTOGRAPHS
Five patients (23.8%) reported adverse event(AE)(Table 2). The most frequently reported AE was diarrhoea (3 patients, 14.28%), followed by headache (2 patients, 9.52%)and Joint pain (2 patients, 9.52%).Table 2 provides an overview of all adverse events during apremilast treatment Table 2: Adverse events (AE) attributed to the drug in 21 psoriasis patients treated with apremilast
DISCUSSION Real-life treatment outcomes may differ from clinical trial results due to preselected patient cohorts in clinical trials. Thus, it is critical to also evaluate efficacy and safety in everyday practice. Such data give us valuable information and can impact our therapeutic regimen.In this study, we report our experiences on efficacy and AEs in psoriasis patients treated with apremilast. Limitations of our study are the non-comparative study design, the modest patient numbers and the fact that many patients were still on treatment at lock date, which may alter outcome. Also, the use of concomitant topical treatment was not taken into account when calculating efficacy. Indeed, the patients’ baseline demographics in our cohort were different from trial patients: (i) Patients of this study were younger than in the two main apremilast trials ESTEEM 1 and ESTEEM 2 (median age: 40 vs 45 years), had lower mean PASI scores (10.7 vs 18.7 and 18.9, in ESTEEM 1 and ESTEEM2 cohorts, respectively).19,22 These differences might be due to the strict inclusion criteria for patien ts who participated in the two clinical trials ESTEEM1 and ESTEEM2. Most patients in our cohort had at least one AE (23.8%), which is lower than numbers reported in ESTEEM 1 and 2 trials (79% and 78%). Such differences between real-life and trial patients are not uncommon and probably due to more rigorous follow-up schedules, methods of data acquisition and a different mindset of physicians and patients, during clinical trials, with a stronger focus on possible AE. This is also evidenced by high numbers of AEs reported in placebo-treated patients, p.e. 57% in the ESTEEM 1 trail. Similar to results from trial patients, diarrhoea and headache were the most frequently reported AEs in our cohort. In summary, apremilast is a safe and valuable therapeutic modality for patients with psoriasis. Its benefits are easy patient management, per os delivery of the active compound and the fact that no pre-treatment laboratory tests are required. As per this study, up to 70% of the patients will reach PASI 50 or better. Further studies are needed to identify patients who are most likely to benefit from apremilast treatment.
REFERENCES
Policy for Articles with Open Access: Authors who publish with MedPulse International Journal of Community Medicine (Print ISSN: 2579-0862) (Online ISSN: 2636-4743) agree to the following terms: Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution License that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal. Authors are permitted and encouraged to post links to their work online (e.g., in institutional repositories or on their website) prior to and during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work.
|
|
 Home
Home