|
Table of Content - Volume 20 Issue 2 - November 2021
Study of Prevalence of Rifampicin resistance among cases of pulmonary Tuberculosis at tertiary care center
Anjali Surykant Deshmukh1, Sanjiv Vithalrao Zangde2*
1Assistant Professor, Department of Medicine, 2Assistant professor, Department of Pulmonary Medicine, Dr Shankarrao Chavan Government Medical College, Nanded, Maharashtra, INDIA. Email: drsanjivzangde98@gmail.com
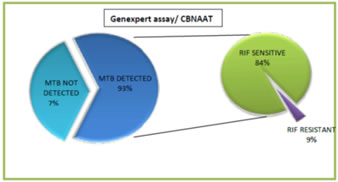
Abstract Background: The advent of multidrug-resistant tuberculosis (MDR-TB), defined as resistance to at least rifampicin and isoniazid, as well as rifampicin-resistant tuberculosis (RR-TB), has posed a significant challenge to global TB control. Aims and Objectives: To Study Prevalence of Rifampicin resistance among cases of pulmonary Tuberculosis at tertiary care center. Methodology: The present study is an observational cross-sectional study of the patients with pulmonary tuberculosis attending Department of Pulmonary Medicine at a tertiary care centre during period of 1 January 2019 to 30 June 2020 by the permission of Institutional Ethical Committee Genexpert test / CBNAAT [ Xpert MTB/RIF Assay] Statistical analysis done buy of (version 20) for Windows package (SPSS Science, Chicago, IL, USA). Result: Majority of the study population were in the age group of 21- 30 years, followed by 31-40 years, the least recorded study subjects were in the age group of 51-60 years. Among all 200 cases GeneXpert assay/ CBNAAT was positive for MTB in 186 (93%) patients, among 186 MTB detected 168 (84%) were Rifampicin sensitive while 18 (9%) were Rifampicin Resistant and 14(7%) patients had Negative GeneXpert assay results. Conclusion: In our study the Prevalence of Rifampicin resistance found to be 7% hence GeneXpert assay found to be very useful for the detection of Refampicin resistance Key words: Rifampicin resistance, GeneXpert assay, MTB.
INTRODUCTION The advent of multidrug-resistant tuberculosis (MDR-TB), defined as resistance to at least rifampicin and isoniazid, as well as rifampicin-resistant tuberculosis (RR-TB), has posed a significant challenge to global TB control.1 According to a World Health Organization (WHO) report, 580,000 new cases of MDR/RR-TB were reported in 2015, with 250,000 MDR/RR-TB patients dying. 2 According to surveillance data, MDR/RR-TB was found in roughly 4% of new TB cases and 21% of previously treated TB cases in 2015.3 India accounts for roughly a third of global TB cases and has the highest number of newly reported TB and MDR-TB patients.4 Various studies in India have reported prevalence rates of MDR-TB ranging from 0.6 percent to 24 percent in new TB cases.5-7
METHODOLOGY The present study is an observational cross-sectional study of the patients with pulmonary tuberculosis attending Department of Pulmonary Medicine at a tertiary care centre during period of 1 January 2019 to 30 June 2020 by the permission of Institutional Ethical Committee The sample size: Total participant of the study were 200. Sample size calculated by formula.80
From previous year hospital data and the previous study literature, the prevalence of pulmonary tuberculosis ranges from 60-66%. To obtain maximum sample size the prevalence was taken as 66%. N = required sample size Z (1-α/2) = 1.96(at 5% type 1 error) p = Expected proportion in population based on previous or pilot study i.e. 66 q = (1-p) i.e. 34 d= absolute error or precision to be decided by researcher i.e., here 10% of the prevalence i.e., 6.6 Hence, N = (3.84x66x34) / (6.6x6.6) = 198 Age > 13 years , All patients attending OPD and IPD with signs and symptoms pulmonary tuberculosis, Chest X-ray findings suggestive of pulmonary tuberculosis, All cases of clinically diagnosed sputum smear-negative pulmonary tuberculosis were included into the study while All the patients with age less than 13 years, Samples other than sputum samples, macroscopically resembling saliva were excluded, Patient who are under Antitubercular therapy for pulmonary, Extra pulmonary and MDR TB were excluded from study, Patients with past history of tuberculosis or treated with a course of anti-tubercular drug therapy were excluded from the study. Sputum for Zeil-Nelson’s stain, Gram stain and culture –sensitivity were carried out. Genexpert test / CBNAAT [ Xpert MTB/RIF Assay] Genexpert test is a rapidly performing cartridge-based nucleic acid amplification test (CBNAAT) which has utility for the molecular detection of Mycobacteria along with the detection of rifampicin sensitivity status. Statistical analysis done buy of (version 20) for Windows package (SPSS Science, Chicago, IL, USA).
RESULT TABLE 1: SHOWING AGE AND SEX GROUP DISTRIBUTION OF PULMONARY TUBERCULOSIS
Majority of the study population were in the age group of 21- 30 years, followed by 31-40 years, the least recorded study subjects were in the age group of 51-60 years.
Table 2: Genexpert assay/ CBNAAT (n= 200)
Among all 200 cases GeneXpert assay/ CBNAAT was positive for MTB in 186 (93%) patients, among 186 MTB DETECTED 168 (84%) were Rifampicin sensitive while 18 (9%) were Rifampicin Resistant and 14(7%) patients had Negative GeneXpert assay results
Graph 1: Pie of pie chart with result of genexpert assay including rifampicin sensitivity
DISCUSSION Demographic profile of present study compared with various other studies as under Majority of the study population were in the age group of 21- 30 years, followed by 31-40 years, the least recorded study subjects were in the age group of 51-60 years. Table 3
Table 4
The proportion of MDR TB patients who had previously consumed ATT ranged from 8% to 67 percent.8-11 According to a recent meta-analysis, MDR-TB cases climbed from 4.1 percent of all new cases in 1995-2005 to 5.6 percent in 2006-2015. The majority of these research used culture as a DST approach.12 In Our study the among all 200 cases GeneXpert assay/ CBNAAT was positive for MTB in 186 (93%) patients, among 186 MTB DETECTED 168 (84%) were Rifampicin sensitive while 18 (9%) were Rifampicin Resistant and 14(7%) patients had Negative GeneXpert assay results The lack of uniform surveillance procedures across India is evidenced by the vast range of resistance prevalence in different sections of the nation. Recognizing this need, the Revised National Tuberculosis Programme (RNTCP) undertook the first national drug resistance survey from 2014 to 2016.14 It recorded the prevalence of any Isoniazid resistance and MDR strains in new PTB cases (n=3065) to be 11.6% and 2.84% respectively. In our study, the prevalence of Isoniazid resistance was the same but MDR was almost three-times. This could be due to our laboratory being a referral center and subsequent referral bias.
CONCLUSION In our study the Prevalence of Rifampicin resistance found to be 7% hence GeneXpert assay found to be very useful for the detection of Refampicin resistance
REFERENCES
Policy for Articles with Open Access
|
|
 Home
Home