|
Table of Content - Volume 4 Issue 2- November 2016
Genotypic diagnosis of extra pulmonary tuberculosis – CBNAAT a novel tool
Gour Sanjay M1*, Munje Radha P2, Ninu P Babu3, Muley Sushant4, Atram Jitesh4
1Associate Professor, 2Professor and HOD, 3Jr. Resident, 4Assistant Professor, Department of Respiratory Medicine, Indira Gandhi Government Medical College, Nagpur, Maharashtra, INDIA. Email: dr_smg2004@yahoo.com
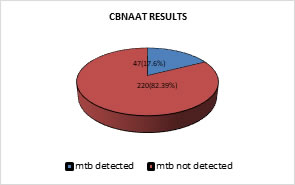
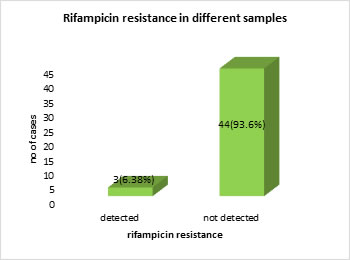
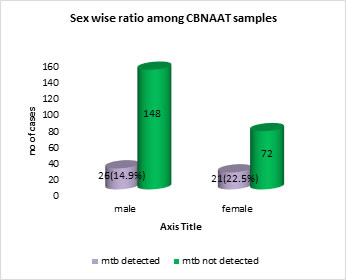
Abstract Objectives: To evaluate the utility of CBNAAT in detecting MTB in suspected extrapulmonary tuberculosis smear negative cases. To see for Rifampicin resistance amongst CBNAAT MTB positive cases. Methods: Retrospective record based analysis. Study period: 6 months October 2016 to march 2017. Results: Retrospective analysis of 267 samples done. Most of the cases were of age group 20 to 50 years of age. Maximum cases were in the age group 21-30 years of age with MTB detected in 13(16.88%) cases. MTB was detected in 47(17.6% )samples. Of these 47 samples, 44(93.6%) were sensitive to rifampicin and 3(6.38%) were resistant to rifampicin. 174(65.16%) were males and 93(34.83%) were females. Of 174 males, MTB was detected in 26(14.9%) and out of 93 females, MTB was detected in 21(22.5%). Conclusion: CBNAAT is a useful test to confirm presence of MTB with simultaneous detection of rifampicin resistance in EPTB AFB smear negative cases. This has an impact on treatment and outcome as all presumptive cases have a confirmed diagnosis. Key Words: Genotypic diagnosis, pulmonary tuberculosis.
INTRODUCTION Tuberculosis (TB) is an important health problem in developing countries like India and remains a key challenge to public health due to inadequate diagnostic assays. Diagnosis of Extra Pulmonary TB (EPTB) still remains challenging as the number of Mycobacterium Tuberculosis Bacilli (MTB) present at the site of diseased tissue is often low and clinical specimens of deep-seated organs are difficult to obtain1. According to WHO there were 8.6 million new TB cases in 2012 and even 1.3 million TB deaths. Extra-pulmonary tuberculosis (EPTB) accounts for 20% (234 029 cases out of 1183 373) of total burden of tuberculosis globally.2 According to the WHO Tuberculosis report 2015, India, Indonesia and China had the largest number of cases: 23%, 10% and 10% of the total global respectively. Globally an estimated 3.3% of new TB cases and 20% of previously treated cases have MDR-TB (WHO, 2015)3 The X-pert assay has been validated and optimized to diagnose HIV-associated TB and multidrug-resistant TB. WHO strongly recommends widespread use of X-pert for these patients. In 2014, WHO has recommended X-pert over the conventional tests (including conventional microscopy, culture or histopathology) for testing specific non-respiratory specimens (lymph nodes and other tissues) from patients suspected of having EPTB4.
MATERIALS AND METHODS Retrospective observational record based analysis of 267 suspected extra pulmonary tuberculosis smear negative cases done for a period of 6months from October 2016 to march 2017. All samples were sent for CBNAAT and data was analysed.
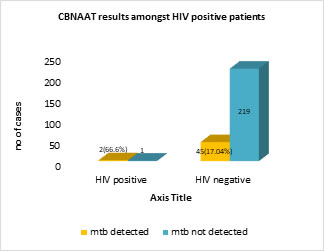
RESULTS Retrospective analysis of 267 samples done. Most of the cases were of age group 20 to 50 years of age. Maximum cases were in the age group 21-30 years of age with MTB detected in 13 (16.88%) cases. MTB was detected in 47(17.6%) samples. Of these 47 samples, 44 (93.6%) were sensitive to rifampicin and 3(6.38%) were resistant to rifampicin. 174 (65.16%) were males and 93 (34.83%) were females. Of 174 males, MTB was detected in 26 (14.9%) and out of 93 females, MTB was detected in 21 (22.5%). Out of 267 patients only 3 were HIV positive. MTB was detected in 2 (66.6%) patients. Out of 264 non HIV patients, MTB was detected in 45 (17.04%). Among different samples processed, maximum cases were of pleural effusion-133(49.81%), of which MTB was detected in 13 (9.77%).
Figure 1:
Figure 2:
Figure 3:
Figure 4:
Figure 5:
Table 1: CBNAAT results among different EPTB samples
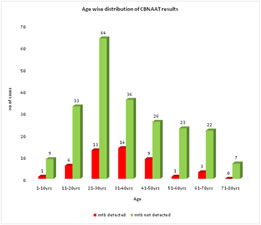
Extra pulmonary tuberculosis constitutes 20% of burden of TB globally. EPTB is a pauci-bacillary disease as the number of bacteria are less to detect and are located deep seated in the organs. Conventional methods like histology and smear microscopy are never diagnostic and diagnostic methods like culture methods are time consuming. Hence there is a need for newer and faster diagnostic methods like nucleic acid amplification techniques like Gene Xpert (CBNAAT).2 V. K. Arora et al conducted a study on trends of EPTB and found higher detection of EPTB cases in younger age group5. Similar results were found in our study. Maximum cases were in the age group 21-30 years, MTB detected in 13 (16.88%) cases. (Image 5) Irfan ullah et al conducted a study on rapid detection of MTB and rifampicin resistance in 168 extra pulmonary tuberculosis samples using CBNAAT. MTB was detected 60 (35.7 %) samples. Sensitivity and specificity of the CBNAAT for detection of TB was 86 and 88.4 % respectively. The positive predictive value was 71.5 % while negative predictive value was 95.1 %6. Lesley Erica Scott conducted a study on diagnostic accuracy of CBNAAT for EPTB specimens. EPTB specimens from hospitalized patients received over a 6-month period were tested using CBNAAT. Culture was used as reference, CBNAAT's overall sensitivity was 59% and specificity was 92%. Highest sensitivities obtained were 91% for pus, 80% for lymph node aspirates, and 51% for fluids (ascitic, 59%; pleural, 47%). It was also found that CBNAAT is less affected by contaminating bacteria reducing diagnostic delay compared to traditional methods7. In our study maximum cases were of pleural effusion-133(49.81%) with MTB detected in 13(9.77%). Other samples processed were 20 cases of lymph node (LN) aspirate with MTB detected in 8(40%), 50 pus samples with MTB detected in 20(37.73%) and other fluids (ascitic, endometrial, synovial, cerebro spinal, pericardial) with MTB detected in 5 out of 58(8.62%). (Table 1) In a study conducted by John K Lusiba, the X-pert MTB/Rif test identified 25 (28.7%) of the 87 confirmed pleural TB cases. The sensitivity and specificity of the test were 28.7% and 96.6% respectively while the positive and negative predictive values were 96.1% and 31.1% respectively.8 Another study was conducted by Mahim mittal et al on comparison of diagnostic yield of CBNAAT and ZN (Ziehl-Neelsen) staining in serosal fluids from HIV and non-HIV patients with extra-pulmonary tuberculosis. 81 extra pulmonary samples (21 from HIV and 60 from non-HIV patients) processed including 48 CSF, 19 pleural fluids and 14 ascitic fluid. 28(34.5%) were both CBNAAT and ZN stain positive while only 11(13.58%) were ZN stain positive. Out of 21 HIV patients 4(19.05%) were ZN stain positive and 9(42.85%) were GeneXpert positive. Among 60 non-HIV patients 7(11.66%) were ZN stain positive and 19 (31.66%) were GeneXpert positive. It was found that CBNAAT is more sensitive than ZN staining in EPTB while both HIV and non-HIV patients has same yield.9 In our study MTB was detected in 2 out of 3(66.6%) HIV patients while in non HIV patients, MTB detected in 45 out of 219(17.04%). The specificity of the test was found to be 99.5%. (Image 4)
CONCLUSION CBNAAT is a useful test to confirm presence of MTB with simultaneous detection of rifampicin resistance in EPTB AFB smear negative cases. This has an impact on treatment and outcome as most presumptive cases have a confirmed diagnosis.
REFERENCE
Policy for Articles with Open Access
|
|
 Home
Home