|
Table of Content - Volume 19 Issue 1- July 2021
A study of prevalence of hypothyroxinemia in low birth weight babies at a tertiary health care centre
Shivprasad Kachrulal Mundada1, Nitin Narayan Ambekar2*
1Professor and HOD, 2PG Resident, Department of Pediatrics, Vilasrao Deshmukh Government Institute of Medical Sciences, Latur, Maharashtra, INDIA. Email: ambekar.nitin214@gmail.com
Abstract Background: Thyroid is one of the most important endocrine glands in human body. Thyroid hormone play an important role in brain development in neonates. Low birth weight neonates are more prone for development of hypothyroxinemia. Aim and objective: To find the prevalence of hypothyroxinemia among low birth weight neonates Methodology: Present study was a hospital based cross sectional study carried out on 135 neonates. Study population was divided in two groups as SGA and AGA and prevalence of decreased T3, T4 and increased TSH was compared in these groups. Results: Prevalence of decreased T3 in study population was 28.14%. Prevalence of hypothyroxinemia (decreased T4) in the study population was 21.4%. It was higher in SGA newborns (35.18%) compared to AGA newborns (12.34%). Prevalence of increased TSH in the study population was13.33%. it was higher in SGA newborns (22.22%) compared to AGA newborns (7.4%)
INTRODUCTION Thyroid hormones play an important role in growth and neurodevelopment. Importance of thyroid hormone concentrations in early postnatal period in neurodevelopment has long been recognized. Congenital hypothyroidism remains most important cause of preventable mental retardation. However, because signs and symptoms of congenital hypothyroidism are non-specific and not easily identifiable a high suspicion is necessary for its diagnosis.1 Routine screening of newborns is necessary to avoid missed diagnosis of congenital hypothyroidism. In developing countries where resources are limited it becomes important to recognize high risk population within newborns so that screening of selective neonates can be done. It is unclear whether thyroid hormones play a role in growth restriction in low birth weight newborns which include both preterm and small for gestational age (SGA) newborns. Very few studies have analyzed thyroid function in SGA and preterm newborns. Studies that have analyzed thyroid function in fetus reported that, T4 and FT4 values in SGA fetuses were significantly lower than AGA fetuses and T3 values in SGA and AGA were not statistically significant. 2,3 Conflicting results were found with respect to TSH values in SGA and AGA results. Very few studies have analyzed thyroid function in SGA and preterm newborns postnatally. TSH levels in SGA newborns are higher compared to AGA newborns. 5,6 Some studies have noted decreased T4 concentration in SGA newborns in early neonatal period, 1,6 while others have noted that there are no difference in T3 and T4 concentrations.5,7 Present study was conducted to find the prevalence of hypothyroxinemia among low birth weight neonates Aim and objective: To find the prevalence of hypothyroxinemia among low birth weight neonates
MATERIAL AND METHODS Present study was a Hospital based Cross sectional analytical study carried out at Level III NICU at Tertiary care centre over study period of 18 months. The study population included all the low birth weight neonates (<2Kg) admitted at a tertiary care centre. Inclusion criteria: 1. All 3 day old neonates with birth weight <2 kg Exclusion criteria: 1.Neonates whose parents are not willing 2. Neonates who were discharged against medical advice Written approval from Institutional Ethics committee was obtained beforehand. Biochemistry, OBGY, Pathology departments were informed about the study to ensure investigation and lab reports. Written approval of Biochemistry, OBGY and Pathology department was obtained. After obtaining written approval study was undertaken by interviewing parents of neonates less than 2 kg weight at birth on 3rd day admitted at a tertiary care centre with the help of questionnaire. A pilot study was conducted on 14 patients in which prevalence of hypothyroxinemia was found 42.85% Sample size was calculated as 134 babies according to formula 8 Sample size= Z2PQ/ L2 Predesigned and pretested questionnaire was used to record the necessary information. Questionnaires included general information, age, sex, religion, Antenatal history, perinatal history, post-natal history, past medical history, advised investigations of baby for T3, T4, TSH, Serum Calcium, Serum Bilirubin, Random Blood Sugar. The interview technique was used as a tool for data collection. After obtaining thyroid function levels, all newborns were divided into two groups in the form of SGA and AGA. Thyroid functions were compared in these two groups. Term SGA was used to describe newborns with birth weight < 2 [S.D.] standard deviation below the mean for the infants GA based on data derived from an appropriate reference population. Hypothyroxinemia was defined as serum T4 concentrations below normal range with TSH levels within the normal range.9 Reference range for T3 was 75-260 ng/dl and for T4 was 8.2 to 19.9 µg/dl. 177 we have taken self definition of increased TSH as TSH >12mIU/L as TSH >12 mIU/L is associated with poor neurodevelopmental outcome.10 The data were entered in Microsoft Excel and data analysis was done by using SPSS for windows. The analysis was performed by using percentages in frequency tables and association of the other determinants related to hypothyroxinaemia. p<0.05 was considered as level of significance using the Chi-square test.
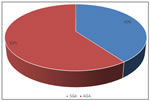
RESULTS Total number of admissions during study period were 2565 among them 135 met inclusion criteria. Out of 135 newborns 6 (4.4%) were < 30 weeks of age, 65 (48.1%) newborns were between 30 to 35 weeks. 64 (47.4%) newborns were between 36 to 40 weeks of gestational age. (table 1) In our study majority of the neonates were with birth weight of 1.5-2kg (56.3%) followed by 1.2- 1.5 kg (36.3%). Babies with birth weight of 1-1.19kg were 6.66%. one baby was with birth weight of less than 1 kg . (table 2) Out of 135 neonates 54 (40%) were small for gestational age and 81 were appropriate for gestational age. (fig 1) Table 3 shows prevalence of decreased T3. In SGA newborns decreased T3 (<75) was found in 21 out of 54 patients (38%) compare to AGA in which T3 was decreased in 17 out of 81 patients (20.9%). The difference was statistically significant (p value 0.023). Table 4 shows prevalence of hypothyroxinemia in SGA vs AGA newborns. In SGA newborns 19 out of 54 had decreased T4 (35.18%) compared to AGA newborns in which T4 was decreased in 10 out of 81 patients (12.34%). The difference was statistically significant with p value 0.0015. Overall prevalence was 21.48%. Overall prevalence of hypothyroxinemia in entire study population that is LBW babies was 38.51% (52 out of 135). Table 5 shows prevalence of increased TSH in SGA vs AGA neonates. Prevalence in SGA was 22.22% (12 out of 54). Prevalence in AGA was 7.4% (6 out of 81). Difference was statistically significant. p value 0.013. Prevalence in overall low birth weight babies was 13.33% (18 out of 135).
Table 1: Distribution of neonates according to gestational weeks
Table 2: Distribution of neonates according to birth weight
Figure 1: Distribution of neonates according to gestational age and birth weight
Table 3: Distribution of neonates according to T3 level and SGA and AGA group
Table 4: Distribution of neonates according to T4 level and SGA and AGA group
Table 5: Distribution of neonates according to TSH level and SGA and AGA group
DISCUSSION Compared to other hormones thyroid hormone in SGA newborns has not been investigated adequately. To our knowledge 3 studies examined thyroid hormone in SGA fetuses 2,3,11, Previous studies have evaluated thyroid hormone at birth and and in early neonatal period.4-7, 12,13 Most of these studies have examined only T4 and TSH in the neonatal period except cartault et al. 5 and PS Pal et al. 7 who have measured T3 along with T4 and TSH. However, results published are conflicting. In present study prevalence of hypothyroxinemia in overall LBW population was found to be 21.4% with prevalence in SGA 35.18% and in AGA 12.34%. Prevalence of decreased T3 was 28.14% in all newborns with 38% in SGA and 20.9% in AGA. Prevalence of both decreased T3 and T4 was significantly higher in SGA newborns compared to AGA newborns. These findings can be compared with PS Pal et al. (2017) 7 who observed that the prevalence of decreased T4 in SGA and preterm AGA was 20%. However, they reported that difference in prevalence of hypothyroxinemia in AGA and SGA newborns was not statistically significant. Possible explanation for this difference in findings may be comparatively small sample size studied by PS PAL et al. 7 and their study included term AGA newborns also. Our study which only included neonates less than 2 kg, all the AGA newborns were preterm. Prevalence of increased TSH in our study was13.33%in SGA vs 7.4% in AGA. This is in line with PS Pal et al. who observed that the prevalence of raised TSH was 11.66% with 20% in term SGA patients.7 In our study mean T3 and mean T4 values were significantly lower in SGA newborns compared to AGA newborns and mean TSH values were significantly higher in SGA neonates compared to AGA group. These findings can be compared with Bagnoli et al. 6 who observed that TSH values were significantly higher in SGA newborns compared to AGA newborns and T4 values were significantly low in SGA newborns compared to AGA newborns. PS Pal et al. 7 reported that both mean T3 and mean T4 values were significantly lower in SGA newborns compared to AGA newborns and mean TSH values were significantly higher in SGA neonates compared to AGA neonates. Setia et al. (2006) 13 found that cord blood mean T4 values were significantly lower and mean TSH values were significantly higher in SGA newborns compared to AGA newborns at birth. However, Jacobsen et al. 14 found that there was no significant difference in mean TSH and T4 values at 5th hour and 160th hour of life. Similar results were given by Mahajan et al. 12 and Rashmi et al. 15 who reported that there was no significant difference in TSH and T4 values in-between SAG and AGA newborns at birth. Cartault et al. (2006) 5 reported that both TSH and T3 values are significantly increased in SGA neonates compared to AGA neonates on 4th day of life. Present study can be compared with Jacobsen et al., 14 bagnoli et al. 6 and PS Pal et al. 7, as these studies have examined thyroid levels postnatally. Our results are in line with Bagnoli et al. 6 and PS Pal et al.7 Our finding of low T3, T4 and high TSH values in SGA newborns indicate reduced T4 secretion from thyroid gland due to persistence of reduced T4 of fetal thyroid physiology till early neonatal period. Possible explanation for this is intra uterine growth restriction leading to decreased nutrients supply and incomplete development of thyroid gland.6 Initially it was believed that antenatal decreased placental circulation leading to hypoxia simulates postnatal starvation in which, hypothyroxinemia serves as a beneficial adaptation. It decreases BMR and thereby O2 requirement. But in postnatal starvation TSH is normal or decreased and repose of pituitary to TRH is also decreased. That is not the case in IUGR in which TSH is raised. This suggests that hypothyroxinemia in SGA fetuses and newborns is not an adaptation to the hazardous environment.2 Another explanation for this hypothyroxinemia in SGA newborns is developmental delay in which development of thyroid gland in preterm and SGA fetuses is delayed. 16 And therefore high TSH may be a result of decreased thyroid hormones. Alternatively, hyperactivity of pituitary due to altered metabolic environment in these SGA fetuses may also lead to increased TSH. Catacholamines have shown to regulate TSH secretion from pituitary.17 A study conducted by Greenough et al. showed that norepinephrine measured by cordocentesis is significantly high in SGA fetuses than AGA fetuses.18 Forth probable explanation for this hypothyroxinemia is in SGA fetuses there is increased brain perfusion with decreased perfusion to thyroid gland along with other organs. Therefore, increased brain perfusion may lead to increased pituitary activity and increased TSH and decreased thyroid perfusion may lead to decreased T4 secretion.2 Another hypothesis that supports this finding of decreased T4, T3 in SGA newborns is deficiency of essential amino acids phenylalanine and tyrosine due to inadequate nutrition leading to insufficient thyroid hormone production.6
CONCLUSION Thyroid function is significantly deranged in low birth weight neonates. SGA neonates have significantly lower T3, T4 levels and significantly higher TSH levels.
REFERENCES
Policy for Articles with Open Access
|
|
 Home
Home