|
Table of Content - Volume 19 Issue 3 - September 2021
Prevalence and predictors of urinary tract infection in children with cerebral palsy
Sambhaji Chate1, Sunil Holikar2, Avinash Sanap3*
1Professor, 2Associate Professor, 3Junior Resident, Department of Pediatrics, Swami Ramanand Teerth Rural Government Medical College, Ambajogai, Beed, Maharashtra, INDIA. Email: avinashsanap26@gmail.com
Abstract Background: Urinary tract infection (UTI) in children with cerebral palsy (CP) is a challenging yet common clinical condition. Aim of present study was to determine the prevalence of UTI, asymptomatic bacteriuria, antimicrobial sensitivity pattern and predictors of UTI among children with CP. Method: A total of 62 patients of age 2 to 18 years were studied in the Department of Pediatrics, at Tertiary care hospital in central India over a period of 24 months from November 2018 to October 2020. Results: The prevalence of UTI and asymptomatic bacteruria among children with CP was 32.25% and 4.8% respectively. Escherichia coli (45%)and Strept Faecalis (20%)were found to be common causative agent of UTI. The major isolates, Escherichia coli, were 100% sensitive to ceftriaxone, amoxiclav, nitrofurantoin, cotrimoxazole, ciprofloxacin, ofloxacin and sparfloxacin but less sensitive to gentamicin (66.66%) and streptomycin (55.55%) while resistant to tetracycline (0%) and nalidixic acid (0%). In bivariate analysis, older children (>5 years), moderate to severe motor disability, dysuria, constipation, flank/abdominal pain, significant haematuria, significant pyuria and significant microscopic pyuria, were found to be significantly associated with UTI (𝑃 values<0.05). In univariate regression analysis only moderate to severe gross motor dysfunction predicts the risk of UTI (OR = 53.72, 95% CI, 2.31-1332.00, 𝑃 value 0.018). Conclusion It is important to screen all the CP children presenting to the hospital (both indoor and outdoor departments) for urine examination as a baseline investigation and also regular follow up required to minimize the urinary complications. Keywords: Urinary tract infection; Cerebral palsy; Bacteriuria; Predictors; Antimicrobials; Sensitivity; Escherichia coli; Gross motor dysfunction
INTRODUCTION Cerebral palsy is a group of permanent, but not unchanging, disorders of movement and/or posture and of motor function that appears during infancy or early childhood resulting from damage to the brain. The damage to the brain is permanent and cannot be cured but the earlier we start with intervention the more improvement can be made.1 Any non-progressive central nervous system (CNS) injury occurring during the first 2 (some say 5) years of life is considered to be CP. Globally, studies have reported the prevalence range of CP from 1.5 to 4 per 1000 live births or children.2,3 while in India, the estimated incidence of cerebral palsy is around 3/1000 live births.4 Moreover, children with CP bare the greatest risk of contracting UTI and the factors that increase the likelihood of acquiring UTI include delay to attain bladder and bowel control, difficulty in neuromotor control of posture, low cognition, limited ability to communicate the need to void, constipation, impaired mobility, and bladder dysfunction.5,6 All these are common findings among children with CP. The burden of UTIs among children with CP in the developed world ranges from 8.5% to 56.7%.7-9 Population-based studies from around the world report the most common causative pathogens for UTI include Escherichia coli, Proteus spp, Enterococcus faecalis, Klebsiella spp, and Staphylococcus spp.7,8 The recommended treatments for these pathogens are amoxiclav and co-trimoxazole.9 The present study was designed with an objective to determine the prevalence of UTI and incidence of asymptomatic bacteriuria as well as detection of most common organisms causing UTI, the antimicrobial sensitivity pattern and predictors of UTI among children with CP.
MATERIALS AND METHODS This observational study was conducted in total 62 patients of age 2 to 18 years presented with or without urinary symptoms and diagnosed with CP in the Department of Paediatrics, at Tertiary care hospital in central India over a period of 24 months from November 2018 to October 2020. Patients who have received antibiotics within 2 weeks prior to enrolment, patients with vaginal/penile discharge or vaginal bleeding and those parents not willing to participate in study were excluded. All information regarding patient age, sex, socioeconomic class and various predisposing factors like instrumentation of the urethra, voiding difficulties were collected. A complete history related to the onset, duration of fever and associated symptoms such as nausea, vomiting, diarrhoea, urinary disturbances, other system involvement was obtained. The assessment of nutritional status was done using the IAP (Indian Academy of Paediatrics) classification for malnutrition. Assessment of Motor Impairment: This Gross Motor Function Classification System (GMFCS) has been used. A complete physical examination with significant investigations was carried out in all children. The blood investigations and urine analysis along with urine culture and sensitivity were done in all these children. The investigations were performed including complete haemogram, erythrocyte sedimentation rate (ESR), C reactive protein (CRP), renal function test (RFT), urine collection, foley’s catheterization, spot urine dipstick test For Leukocyte Esterase and Nitrates, urine routine microscopy and ultrasonography of the urinary tract. Collection of urine sample Urine samples were collected from all the 62 children. In children less than 2 years of age urine was collected by a bag collection method and in children above 2 years clean midstream sample was collected. All urine samples were sent to lab within 1 hour or if delay can preserve sample at 4°c for about 12-24hrs in refrigerator Method of urine analysis The urine samples obtained from the above techniques were then subjected for urinalysis and urine culture and sensitivity. The urine specimens were then centrifuged in a chamber, 10ml of urine was span at the rate of 2500 rpm for about 30 minutes, and the supernatant fluid was then decanted off and the remaining sediment was resuspended in the chamber. The urine was then examined under microscope for Hematuria, and Leukocyturia. In our study more than 5 pus cells /HPF in a centrifuged sample of urine was considered as significant pyuria and culture and sensitivity was performed in that child. Method of urine culture The clean mid-stream catch urine was inoculated into blood and mac conkey agar plates using a 0.01millilitre calibrated loop. All plates were then incubated at 35-370C for about 24 hours under aerobic condition in order to obtain accurate colony count. On culture of the mid-stream sample of urine, a colony count of more than 105 /ml organisms of a single species of bacteria were considered to be significant. Samples with insignificant growth, mixed growth of two or more pathogens or growth of non-pathogens were not considered to be culture positive. Positive urine culture A positive urine culture was defined as growth of >105 colonies of a single urinary tract pathogen/ml of specimen in a clean mid-stream of urine. For the purpose of this study, the following definitions were applied:
Statistical Analysis
OBSERVATIONS AND RESULTS Among the total 62 patients, 35(56.45%) patients were males and 27 (43.54%) were females. 70.96% children of CP were found in the age group of 2-10 years with mean age of patients was 8.53±3.83 years, ranged from 2-18 years. However, the distribution of age group of CP cases was statistically similar in male and female with p value of 0.421 as shown in table 1. Table 1: Distribution of the Study subjects according to Age and gender
From the table 2, it was observed that spastic CP was the commonest physiological type of CP 56 (90.32%). Among the spastic group, quadriplegia was 53.22%, diplegia 22.58%, double hemiplegia 12.90% and monoplegia 1.61%. The malnutrition was more with female (92.59%) compared to male (85.71%) with p value of 0.234. Majority of children were grade I (46.77%) malnourished followed by grade II malnutrition (29.03%) whereas 11.29% patients had normal nutritional status. The prematurity was (54.83%) commonest cause of CP.
Table 2: Clinical type of CP, Nutrition status of patients and causes of CP
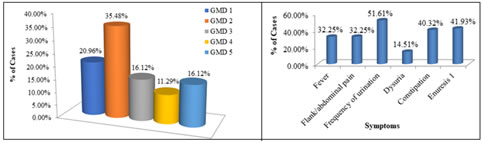
Regarding the degree of gross motor dysfunction, maximum patients (35; 56.45%) had mild dysfunction including 13 (20.96%) with grade 1 and 22 (35.48%) with grade 2. Moderate to severe motor dysfunction was seen in 27 (43.54%) including 10(16.12%) with grade 3, 07 (11.29%) with grade 4, and 10 (16.12%) with grade 5 as depicted in figure 1. For intellectual disability Binet Kamat Test of Intelligence was used, among the 54 children with CP whose ages were between 3 and 18 years, maximum number of children had mild intellectual disability 28 (51.85%) followed by moderate intellectual disability 16 (29.62%) and 10 children had severe disability (18.51%). Figure 1: Distribution of cases according gross motor dysfunction Figure 2: Distribution of patients according to presenting symptoms Most of the patients had symptom of frequency of urination 32 (51.61%) followed by history of constipation and enuresis as shown in figure 2. Table 3 presented urinalyses findings among subjects with CP and which showed urine culture proven UTI in 20 cases. Thus, the prevalence of UTI among children with CP was 32.25%, of them 20.96% (13 cases) were male and 11.29% (7 cases) were female. The maximum number of UTI positive cases were observed in 5-7 years age group (25%) followed by 8-10 years (20%). Table 3: Urinalyses findings among subjects with cerebral palsy
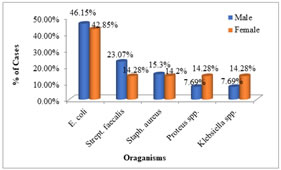
Out of 62 cases, 3 cases had asymptomatic bacteriuria. Thus, the prevalence of asymptomatic bacteruria in children with CP was 4.8%. Of the 07 females examined (UTI positive), 2 (28.57%) had AUTI while 1 (7.69%) of the 13 males had significant bacteriuria. The most common organisms causing UTI in CP children was escherichia coli (45%) followed by Strept. Faecalis isolated in 20% children. From figure 3 it was observed that the escherichia coli were isolated from majority of male (46.15%) and female (42.85%) subjects.
Figure 3: Organisms causing urinary tract infection in cerebral palsy
Table 4 shows the antimicrobial sensitivity pattern of organisms causing UTI in CP. The major isolates i.e. Escherichia coli and Streptococcus faecalis were 100% sensitive to ceftriaxone, amoxiclav, nitrofurantoin, cotrimoxazole, ciprofloxacin, ofloxacin and sparfloxacin but less sensitive to gentamicin and streptomycin while resistant to tetracycline (0%) and nalidixic acid (0%). Table 4: Antimicrobial sensitivity pattern of organisms causing UTI in CP
E. coli: Escherichia coli, Staph. aureus: Staphylococcus aureus, Strept. faecalis: Streptococcus faecalis.
In bivariate analysis, older children (>5 years), moderate to severe motor disability, dysuria, constipation, flank/abdominal pain, significant haematuria, significant pyuria and significant microscopic pyuria, were found to be significantly associated with UTI (𝑃 values <0.05) (Table 5 and 6). However, in univariate regressionanalysis only moderate to severe gross motor dysfunctionpredicts the risk of UTI (OR = 53.72, 95% CI, 2.31-1332.00, 𝑃 value 0.018), (Table 7).
Table 5: Bivariate analysis: Association between some characteristics of subjects with CP and presence of UTI
Table 6: Urinalyses findings among subjects with CP and the relationship with UTI
Table 7: Predictors of UTI among the children with cerebral palsy
DISCUSSION Children with CP suffer from multiple problems and potential disabilities such as mental retardation, epilepsy, feeding difficulties, vision, and hearing impairments. Screening for these conditions should be part of the initial assessment.10 Also, children with CP may present with lower urinary tract dysfunction which increases their risk for UTI and it is a challenging yet common clinical condition. In developed countries special adaptive equipment’s such as walkers, poles, standing frames and electronic wheel chairs are available to help in ambulation of CP children. However, in developing countries like India such facilities are lacking which limits mobility and hence increases the susceptibility to UTI. Furthermore, no local study is published regarding UTI in CP patients and we found an international study reported 38.5% prevalence of UTI in children with CP.22 Hence the present study was conducted in 62 patients to find out the prevalence and predictors of UTI among central Indian children with CP. Urinalyses findings showed that the urine culture proven UTI in 32.25% of cases with CP and this prevalence is under the values reported by developed countries. However, authors from developed countries report an increase of 2.2-32.5% of UTIs among patients with CP.11,12 Few studies reported low prevalence rates of UTI in CP patients13,14; moreover, other several studies documented high prevalence rates.8,11 These disparities may be due to the non-uniformity of study designs and the possibility of different forms of bias in these studies. More importantly, the definition of UTI adopted by the investigators may also have contributed to the absence of unanimity in the findings. Furthermore, the frequency of UTI of 32.25% found in the present study is comparable to the 32.5% reported by Ozturk et al. in Turkey,11 but is much higher than the respective 7.4% and 2.2% reported by Reid and Borzyskowski in London12 and Hellquist et al.15 It was observed that the maximum numbers of our UTI positive cases were (20.96%) males with a male to female ratio of 1.8: 1 and most of were found in 5-7 years age group (25%) followed by 8-10 years (20%), 2-4 years (20%) and 11-13 years (15%). In age group of 14-16 and 17-18 years 10% cases each. These findings are correlated with the previous studies.14,16, and17 Diagnosis of asymptomatic bacteriuria is made by urine culture. Either a properly collected clean-catch specimen or a catheterized specimen is acceptable. The Infectious Diseases Society of America (IDSA) has established criteria for diagnosing asymptomatic bacteriuria.18 Out of 62 cases, 03 cases had asymptomatic bacteriuria. Thus, the prevalence of asymptomatic bacteriuria in children with CP was 4.8%. These findings are comparable with the other studies.8,17Escherichia coli have been shown to account for up to 75% of UTIs in all paediatric age groups followed by Klebsiella spp., Proteus spp., and Pseudomonas spp.19 As may be expected, Escherichia coli were also the commonest isolates (45%) among our subjects with CP. Strept. Faecalis isolated in 20% children, Staphylococcus aureus in 3 (15%), while Proteus spp. and Klebsiella spp. were isolated in 2 (10%) children each. All the 3 children with Staphylococcus aureus isolate also presented with fever. Similar findings are reported in previous studies.7,8,11, and 14 Various studies had shown that Escherichia coli are becoming highly resistant to the first line empirical antimicrobials for UTI, including cotrimoxazole, amoxicillin, nitrofuratoin and nalidixic acid. Its preserved sensitivity to the quinolones and ceftriaxone and gentamicin may be explained by the fact that the quinolones are rarely prescribed for children and the parenteral routes of ceftriaxone and gentamicin reduce the abuse of these two antibiotics. Although in vitro resistance may not necessarily mean in vivo resistance.20,21 In current study, the major isolates, Escherichia coli, were 100% sensitive to ceftriaxone, amoxiclav, nitrofurantoin, cotrimoxazole, ciprofloxacin, ofloxacin and sparfloxacin but less sensitive to gentamicin (66.66%) and Streptomycin (55.55%) as well as were resistant to tetracycline (0%) and nalidixic acid (0%). Streptococcus faecalis were 100% sensitive to ceftriaxone, amoxiclav, nitrofurantoin, ciprofloxacin, ofloxacin and sparfloxacin and less sensitive to gentamicin (75%), streptomycin, cotrimoxazole (50%) but resistant to tetracycline and nalidixic acid. Staphylococcus aureus is 100% sensitive to ceftriaxone, amoxiclav, nitrofurantoin, ciprofloxacin, ofloxacin and sparfloxacin and less sensitive to cotrimoxazole (66.66%), gentamicin (33.33%) and resistant to streptomycin, nalidixic acid and tetracycline. Proteus spp. 100% sensitive to ceftriaxone, nitrofurantoin, ciprofloxacin, ofloxacin and sparfloxacin, less sensitive to gentamicin, streptomycin and amoxiclav (50%), resistant to nalidixic acid tetracycline, cotrimoxazole. Klebsiella spp. 100% sensitive to ceftriaxone, cotrimoxazole, nitrofurantoin, ciprofloxacin, ofloxacin, sparfloxacin and gentamicin, less sensitive to amoxiclav (50%) and resistant to nalidixic acid, streptomycin, tetracycline. These findings are in accordance with the study done by Anígilájé et al.8 and Ryakitimbo. et al.14 Abdominal ultrasound scan among the 20 CP children with UTI revealed no renal parenchymal abnormality. In 3 female children there were bladder wall thickness, irregularity of the bladder wall, and residual urine. Anígilájé et al.8 found similar results. To our knowledge, Silva et al.17 study is the first study to examining ultrasound assessment of BWT in a population with CP. The overall mean BWT was 2.30±0.79 mm. There was also no significant variation in BWT between children with and without LUTD, or between patients who could walk and patients who could not. We found out the predictors of urinary tract infections: In bivariate analysis, older children (>5 years), moderate to severe motor disability, dysurias, constipation, flank/abdominal pain, significant haematuria, significant pyuria and significant microscopic pyuria, were found to be significantly associated with UTI (p values <0.05). However, in univariate regression analysis only moderate to severe gross motor dysfunction predicts the risk of UTI (OR = 53.72, 95% CI, 2.31-1332.00, p value 0.018). These results are comparable with the study conducted by Anígilájé et al.8
CONCLUSION As per findings of present study the frequency of urinary tract infection among children with cerebral palsy was 32.25% and this incidence was significantly higher in male child. The prevalence of asymptomatic bacteriuria in children with cerebral palsy was 4.8%. So, it is important to screen these children for urine examination as a baseline investigation in all the cerebral palsy patients presenting to hospital (both indoor and outdoor departments) and also regular follow up required to minimize the urinary complications.
REFERENCE
Policy for Articles with Open Access
|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 Home
Home