Official Journals By StatPerson Publication
|
Table of Content - Volume 10 Issue 3 -June2019
Preemptive analgesia in patients undergoing infraumbilical laparotomy under spinal anesthesia: A randomized, double-blind, comparative evaluation of varying doses of oral pregabalin
Derlin Thomas1*, Betsy Varghese2, Manu Sam Mathew3, George Kanjirathummoottil George4
1Assistant Professor, 2,3Post Graduate, 4Professor, Department of Anesthesiology, Azeezia Institute of Medical Sciences and Research, Kollam, Kerala, INDIA. Email: derlin.t@gmail.com
Abstract Background: Pain is the foremost complaint in majority of patients following surgery. Even though, several studies have reported the effectiveness of preemptive pregabalin, its optimal dose for reducing acute post-operative pain is still a mystery. Hence, this study was conducted to evaluate the ideal preemptive analgesic dose of pregabalin. Methods: In this prospective, double-blind, parallel-group, randomized trial, 44 patients undergoing spinal anesthesia for infraumbilical laparotomy were randomized into 2 groups: Group A received 75mg of oral pregabalin, whereas Group B received 150mg of oral pregabalin, one hour preceeding spinal anesthesia. Visual Analogue Scale was used for the assessment of pain, post-operatively. Results: The mean time to first VAS> 3 was 540 ± 178.99 min in Group A, while in Group B was 665.71 ± 266.24 min with no statistically significant difference noted. The average dosage of diclofenac given during the first 24 hours post-operatively in Group A was 220.81 ± 45.63 mg and 203.71 ± 51.11 mg in Group B, which was also statistically similar. But a statistically significant increase (P =0.001*) was observed in the mean time taken to two segment regression in sensory blockade, in Group B (174.14±13.9 min) when compared to Group A (157.29±12.06 min). Ramsay sedation score 3 was noticed in eight patients in Group B and only in four patients in Group A. Conclusion: Preemptive oral pregabalin 75mg and 150 mg are equally effective in providing good post-operative analgesia, but pregabalin 75 mg may be an ideal choice considering lesser side effects. Key Word: Preemptive analgesia; Pregabalin; Spinal Anesthesia; Post-operative pain; Visual Analogue Scale; Laparotomy.
INTRODUCTION Pain is a consistent and cardinal complaint of most individuals following surgical interventions. Failure to alleviate pain is morally and ethically intolerable. Effective control of post-operative pain is still a major challenge to the anesthesiologists worldwide and in majority of the cases it is poorly treated1,2. Early post-operative pain is the most common complaint and also one of the prime reasons for prolonged post-surgical convalescence. Consequently, optimal post-operative pain alleviation is vital, as it allows early mobility, better functional recovery, and significant reduction in morbidity and mortality, post-operatively2. Pregabalin is a γ-aminobutyric acid (GABA) analog that binds presynaptically to the α2-δ subunit of voltage-dependent calcium channels, which triggers a decline in neuronal hyperexcitability due to the reduction in the release of several excitatory neurotransmitters3. Numerous studies had been done with different doses of, pre-operatively administered oral pregabalin, showing their effectiveness in mitigating post-operative pain.4-9 But the optimal pre-emptive pain relieving dose of pregabalin still eludes us. This study was therefore designed to find out the ideal dose of oral pregabalin, given pre-emptively for post-operative pain relief, by comparing its varying doses in patients undergoing infraumbilical laparotomy under spinal anesthesia.
MATERIAL AND METHODS After obtaining the Institutional ethics committee approval and getting registered with Clinical Trials Registry-India, 44 patients of either sex, between 18-65 years, of American Society of Anesthesiologists physical status (ASA PS) 1-2, who were scheduled for infraumbilical laparotomy under spinal anesthesia and who were keen and able to render written informed consent, were enrolled in this prospective, parallel group, double-blind, randomized study. Patients with a known drug allergy to pregabalin, ischemic heart disease, uncontrolled hypertension and diabetes mellitus, cerebrovascular disease, renal and hepatic dysfunction, history of alcohol intake and drug abuse, any contraindications for spinal anesthesia, neurological disorders, chronic pain, on NSAIDs and other analgesics, pregnancy and lactation were excluded from this trial. The detailed preanesthetic examination of all enlisted patients, was done one day before the scheduled surgery. A written file containing all the information about the anesthesia technique and the drug to be administered was provided to all the patients. After obtaining patient’s informed written consent, they were made aware of Visual Analogue Scale (VAS) [Table 1] which was used in the post-operative period for the assessment of pain, with its scores extending from 0 (no pain) to 10 (worst imaginable pain)10. Prior to surgery, all patients were fasted for eight hours. Premedications with oral ranitidine 150 mg and oral alprazolam 0.25 mg at night before the surgery were given to all patients. Using a computer-derived randomization method, 44 patients were allocated by means of sealed, sequentially numbered, opaque envelopes into two groups of 22 each. One hour before giving spinal anesthesia: Group A received single administration of 75 mg of oral pregabalin and Group B received single administration of 150 mg of oral pregabalin. After opening the envelopes, the anesthesia resident who was not part of the study administered the drugs (kept in 2 separate bottles labeled A and B) and they did not take part in the further assessment of these recruited patients. The patients were unaware as to which group they belonged to since both the tablets were single and of same colour. After arriving at the operating room, all standard monitors were attached and baseline Heart Rate (HR), SpO2, Systolic Blood Pressure (SBP), Diastolic Blood Pressure (DBP) and Mean Arterial Blood Pressure (MABP) of all patients were recorded, followed by preloading with ringer lactate 15 ml/kg intravenously (IV). Ondansetron 50 mcg/kg IV was also given to all patients. Keeping patient in lateral position at L3-L4 space, under strict aseptic precautions, with hyperbaric bupivacaine 0.5% at 0.3mg/kg, with a maximum dose of 20 mg, using Quincke’s 25 gauge spinal needle subarachnoid block was administered. Oxygen via facemask at 4 L/min were given to all patients and urine output was also recorded. During the entire period of study the patients were monitored for any evidence of:
Post-operatively, after shifting the patient to Post Anesthesia Care Unit (PACU), the following datas were recorded by the staff nurse in PACU, who was unaware of the allocated group of the patient:
If VAS score exceeded 3, all patients were given diclofenac 1 mg/kg IV slowly as rescue medication and if pain didnot come down after 15 min, pentazocine 0.5mg/kg IV was administered. The patients were monitored for VAS score and for any adverse effects like dry mouth, pruritis, headache, dizziness, sedation, visual blurring, nausea and vomiting, 4thhourly during the first 24 hours post-operatively. Sedation scoring was done using Ramsay sedation scale [Table 2]11. Standard protocols were followed in the event of any serious complications. The primary outcome of this study was to estimate the 24 hour total consumption of analgesics and hence to compare the efficacy of varying doses of pregabalin, when given as preemptive analgesia in patients undergoing spinal anesthesia for infraumbilical laparotomy. Whereas secondary outcomes were to determine the time to first requirement for rescue analgesia, recovery time from sensory block, reduction of pain scores post-operatively and tolerability of the drug doses used represented by side effects. Statistical analysis: Sample size was calculated from the study9, with a power of 90% and a significance level of 5%, with P75=1.5±0.51 and P150 =1.09±0.302 and the minimum sample size needed was calculated to be 22 for each group. The statistical calculations were performed using the software Statistical Package for the Social Sciences [SPSS Inc., Chicago, IL, USA] for Windows version 15.0. The following statistical methods were employed in the present study. Proportions and mean were used for descriptive statistics. For proportions chi square test or fisher exact t-test was used and for mean student t test was used. For change in SBP, DBP, MABP and HR repeated measure ANOVA was used. Level of significance was set at 0.05 and all tests were Two -tailed.
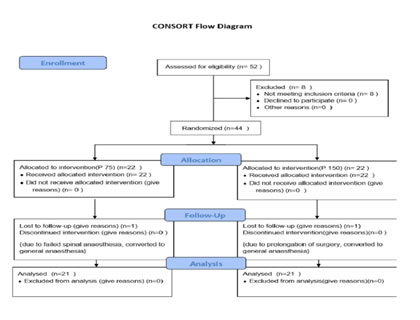
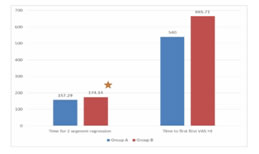
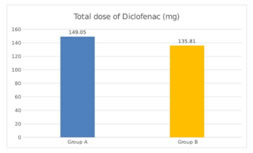
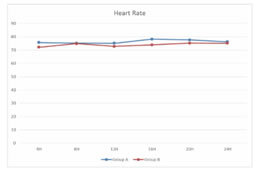
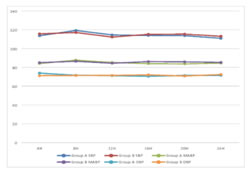
RESULTS We screened 52 patients for this prospective, parallel-group, double-blind, randomized comparative trial. Eight patients were excluded from our study, for not being able to fulfil the inclusion criteria; three patients had ASA PS grade >2, two patients were having history of alcohol abuse, two patients were already on medications with NSAIDs and one patient was not willing for spinal anesthesia. Finally, a total of 44 patients were enlisted and allocated into two groups of 22 each. Among these, one patient from each group discontinued the study, while rest of the 21 patients of both groups completed the study and were subsequently followed up and analysed [Figure 1]. Both groups were comparable in respect of distribution of age, gender, ASA PS, body weight, dose of bupivacaine as well as duration of surgery [Table 3]. Statistically similar observations were noted between Group A (Pregabalin 75) and Group B (Pregabalin150), in the time taken to attain a T6 sensory level after giving spinal anesthesia. Maximum dermatomal level of sensory blockade achieved were also comparable between the groups [Table 4]. Whereas a statistically significant increase (P =0.001*) was observed in the mean time from giving spinal anesthesia to two dermatome regression in sensory blockade in Group B (174.14 ±13.9 min) when compared to Group A (157.29 ±12.06 min) [Table 4, Figure 2]. The mean time to first VAS >3 from the time of giving spinal blockade was (665.71 ±266.24 min) in Pregabalin 150 mg group on comparing with Pregabalin 75mg group (540 ±178.99 min), but no statistically significant difference was noted between the two [Table 4, Figure 2]. The mean of total dosage of diclofenac given during the first 24 hours post-operatively in Group A (P75) and Group B (P150) was 149.05 ± 26.96 mg and 135.81 ± 34.08 mg respectively, which were statistically similar [Table 4, Figure 3]. The mean of total number of doses of diclofenac given during 24 hours in Group A was 2.14, whereas in Group B was 1.90 with no statistically significant difference noticed [Table 4]. Two patients in both the groups needed additional analgesia with opioids [Table 4]. Majority of the patients of Group A (85.7%) and Group B (81%) had a VAS score > 3 only on two occasions during the first 24 hours of post-operative period. VAS score more than 3 was observed thrice in three patients in Group A, while only one patient in Group B had the same. In Group B 14.3% experienced a VAS score > 3 only once with no one in Group A [Table 4]. There was no statistically significant difference between the two groups. Only one patient [Group B] in our study had intra-op bradycardia which was treated with a bolus dose of inj. atropine. While intra-op hypotension was recorded in 16 patients in Group A (P75) and in 17 patients in Group B (P150), requiring inj. mephentermine and showed statistically similar results. Ramsay sedation score 3 was noticed in eight patients in Group B and only in four patients in Group A. While 17 patients in Group A and 13 patients in Group B had a sedation score of 2. No significant difference was noticed in the analysis of sedation in both the groups. Post-op nausea and vomiting was observed in three patients in Group A and two patients in Group B. None of the patients had pruritis, dry mouth, headache or visual disturbances [Table 5]. No statistically significant difference was noted in the post-operative changes in heart rate, SBP, DBP and MABP in both the groups [Figure 4, Figure 5].
Figure 1 Figure 2: Time 2 segment Regression and time of First VAS score >3 Figure 3: Total dose of diclofenac(mg) Figure 4: Post -operative changes in Heart Rate Figure 5: Post-operative changes SBP, MABP, DBP **significant at the level of P<0.05 using repeated measure ANOVA ***SBP-sytolic Blood Pressure, MABP Mean Arterial BP, BDP-Diastolic BP
Table 1: Scoring of Pain using Visual Analogue Scale (VAS)
Table 2: Ramsay Sedation Scale
Table 4: Comparison of Outcome Parameters
*Significant at the level of P < 0.05 using student t-test/ Chi square test/ Fisher exact T-test ** T6- T6 Dermatomal level, T4 – T4 Dermatomal level, VAS – Visual Analogue Scale
Table 5: Comparison of Side effects
DISCUSSION Pain from operative procedures is produced as a consequence of tissue injury resulting in physical, emotional and cognitive agony and discomfort to the patient. Cellular or tissue damage releases chemical mediators resulting in nociceptor activation, leading to central neuronal sensitization which in turn results in amplification of pain post-operatively12,13. Gamma amino butyric acid analogues such as pregabalin and gabapentin is observed to have antinociceptive and antihyperalgesic effects, which were demonstrated in various experimental models of inflammatory hyperalgesia and neuropathic pain14,15. Among them pregabalin is found to be more effective in the prevention of neuropathic component of acute post-surgical nociceptive pain, producing a higher opioid sparing effect and ameliorating peri-operative anxiety 16. Pregabalin is known to have a largely foreseeable and linear pharmacokinetics, which makes it easier for its use clinically. When given orally pregabalin shows a faster and extensive absorption in a fasting state, with maximum plasma concentration at approximately one hour after a single or multiple administrations, with a steady state achieved in 24-48 hours after repeated dosages1,3. Recently numerous trials had been done, studying extensively the effectiveness of varying doses of oral pregablin in alleviating post-operative pain when given pre emptively, with conflicting results.1,6,8,9,11,17-20 Hence, the optimal preemptive analgesic dose of pregabalin is still a mystery. Since no study had been done comparing oral pregablin dose of 75 mg and 150 mg in patients undergoing spinal anesthesia, we designed this study to find out the optimal dose of pre-operatively given pregabalin for post-operative pain relief in these subjects. As increased incidence of side effects such as dizziness and sedation were noticed with higher doses of pregabalin, we omitted those from our study.6,9,18,21 In our study we observed that the mean time from giving spinal anesthesia to two dermatome regression of sensory block from its highest level, was significantly lengthened in pregabalin 150 mg group when compared to pregabalin 75mg. In infraclavicular nerve blocks the pre-operative use of pregabalin resulted in early motor block along with a prolonged sensory blockade.22 Our findings were also similar to the results obtained by Park and Jeon, where they concluded that pre-operatively administered pregabalin 150 mg prolonged both sensory and motor block in patients undergoing urogenital surgery under spinal anesthesia.23 The mean time taken to first VAS score >3 (period of analgesia) from the time of administering spinal block was slightly prolonged in pregabalin 150 mg group on comparing with pregabalin 75 mg group, but no statistically significant difference was noted. Bafna et al24, after conducting a study in patients having elective gynecological surgeries under spinal anesthesia concluded that preemptive dose of gabapentin 600 mg and pregabalin 150 mg markedly prolonged the period of post-operative analgesia and decreased the total requirement of post-operative rescue analgesics. Also Sebastian et al8, observed marked prolongation of analgesia along with reduced rescue analgesics consumption in the post-operative period in patients undergoing elective ortho surgeries of lower limb under spinal anesthesia, on administering oral pregabalin 150 mg pre-operatively. Agarwal et al in his study also noticed similar results after administering pregabalin 150 mg pre-emptively1. However Paech et al17, in a randomized study assessing single preoperative pregabalin at a dose of 100 mg concluded that it neither decrease acute pain nor improve recovery after minor uterine surgical procedures and asserted that an orally given preemptive pregabalin 100 mg is insufficient in relieving postoperative pain. Our results are not in compliance with these findings as we observed a statistically equivalent prolongation in the duration of analgesia in the post-operative period with 75mg pregabalin when compared to 150mg pregabalin. The total rescue analgesic (diclofenac) consumption in both the groups were lower and showed no statistical difference between the two groups. Eventhough the mean of total number of doses of diclofenac given was slightly higher for pregabalin 75mg group, they were statistically similar. These observations confirmed the findings of similar previously done studies.6,8,24 Only two patients in both groups needed supplementary opioid analgesia. The maximum Ramsay sedation score recorded in this study was 3, which was noticed in eight patients in pregabalin 150 mg group and in four patients in pregabalin 75 mg, with no significant difference between the two. The rest of the patients in both groups had a maximum score of 2. These results are in support of the previous studies6,9,18,21, in which they demonstrated a significantly lesser incidence of side effects like sedation and dizziness with lower doses of pregabalin. Intra-op bradycardia was noted only in one patient (pregabalin 150 mg), while intra-op fall in SBP was recorded in 16 patients in pregabalin 75mg group and 17 patients in the other group, with no statistical difference between the two. This can be ascribed to the effect of spinal anesthesia given, but may need further trials to analyse this finding. No significant difference was noticed in the post-operative changes in heart rate, SBP, DBP and MABP in both the groups. Post-op nausea and vomiting was observed in three patients in pregabalin 75 mg group and two patients in pregabalin 150 mg. None of the patients had pruritis, drymouth, headache or visual disturbances. There are a few limitations in our study. We used only a single administration of oral pregabalin during our study, but we arrived at this by evaluating the findings of previous studies. Also we didnot assess the effect of continuing the therapy and follow up was not continued beyond 24 hours. Further studies are recommended in these areas.
CONCLUSION Pre-operatively administered single dose of oral pregabalin 75mg and 150 mg are equally effective in lengthening the duration of first rescue analgesic medication as well as in reducing the total rescue analgesic consumption, but considering lesser incidence of side effects with pregabalin 75 mg, it may be the optimal pre-emptive analgesic dose for infraumbilical laparotomy under spinal anesthesia. ACKNOWLEDGEMENT We thank Mr. Kevin Suresh, who conducted the statistical analysis of the data of our study. We also express our sincere gratitude to all the patients who participated in the study and to the staff of department of Anesthesiology.
REFERENCES
|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 Home
Home