Official Journals By StatPerson Publication
|
Table of Content - Volume 3 Issue 1 -July2017
Evaluation of efficacy of intravenous magnesium sulphate in general anaesthesia cases for post operative analgesia
Sudeep1, N Gopal Reddy2*
1Associate Professor, 2Professor, Department of Anaesthesiology, Kamineni Institute of Medical Sciences, Narketpally, Nalgonda, Telangana
Abstract Background: Surgical procedures are associated with tissue injury and postoperative pain after surgical interventions. Aim: To evaluate the efficacy of intravenous magnesium sulphate on post operative analgesia following surgeries done under general anaesthesia. Objectives: 1. To evaluate post operative pain at different intervals following the use of preoperative and intraoperative intravenous magnesium sulphate 2. To assess the level of sedation in the immediate post operative period. 3. To monitor hemodynamic parameters perioperatively. Material and Methods: This study was done on 60 patients posted for surgery under general anaesthesia, after fulfilling required formalities. 30 mins before induction of anaesthesia, in Group 1, MgSO4 infusion was administered at the rate of 40mg/kg in 100 ml NS IV over 15 mins and same volume of normal saline was administered in group 2. Results: The mean VAS score postoperatively in magnesium group are at 0,1,2,3,4,8,16,24 hours was 1.96±0.66, 1.46±0.50, 1.20±0.48, 1.33±0.47, 1.43±0.62, 3.00±0.45, 1.70±0.53, 0.86±0.34 respectively. The mean VAS score postoperatively in control group at 0,1,2,3,4,8,16,24 hours 2.56±0.56, 1.96±0.41, 1.76±0.56, 1.96±0.41, 2.30±0.53, 4.16±0.69, 2.36±0.61, 2.00±0.26 respectively. The decrease in pain scores in magnesium group was statistically significant (p<0.05). The mean number of rescue analgesics required postoperatively is lesser in magnesium group compared to that in control group. Conclusion: From the present study it is concluded that IV administration of MgSO4. useful as adjuvant preemptive analgesic, significantly reduces postoperative pain and analgesics requirement.. Key Words: General anaesthesia, Magnesium sulphate, Post –op Analgesia, sedation score, VAS scores.
INTRODUCTION Surgical procedures are associated with tissue injury and majority of patients will experience some degree of postoperative pain after surgical interventions, inspite of conventional pain management.1 Ineffective postoperative pain management may lead to coronary stress, atelectases, pneumonia, poor wound healing, insomnia and demoralization. Effective postoperative analgesia may facilitate recovery and reduce morbidity in surgical patients by blunting autonomic, somatic and endocrine reflexes2.Important goals for postoperative pain management are to minimize discomfort, facilitate the recovery process and avoid complications. One of the intravenous adjuvant that has shown potential in preemptive analgesia is magnesium sulphate3 (MgSO4).
MATERIAL AND METHODS The present clinical study was conducted at teaching medical Institute during the period September 2015 to October 2016. After obtaining approval from institutional ethical committee, the present study was undertaken to evaluate the efficacy of intravenous magnesium sulphate on post operative analgesia following surgeries done under general anaesthesia. It was a prospective control study done on 60 patients undergoing elective surgeries under GA. Inclusion Criteria: ASA grade I and II patients age between 20 – 50 years of either gender under elective surgeries posted under GA. Exclusion Criteria: Patients with hepatic, renal diseases, diabetes mellitus, asthma, chronic obstructive pulmonary disease, any hematological disorders, neurological diseases or cardiovascular diseases like heart blocks, hypertension etc. Patients receiving treatment with calcium channel blockers or Mg. Patients with any known allergy to MgSO4 or other drugs and pregnant women. Methods: After a thorough clinical examination and relevant laboratory investigations of all patients, an informed, valid, written consent was obtained; both for conduct of the study as well as for the administration of GA. Patients were explained preoperatively about VAS scale. All the patients were re-examined, assessed and weighed pre-operatively on the day of surgery. Intravenous access was established with a 18G intravenous cannula and baseline hemodynamic parameters i.e., HR, SBP, DBP, MAP were noted. Anaesthesia machine and accessories were checked. Drugs including emergency drugs were kept ready. Also monitoring equipments like pulse oximeter, non invasive blood pressure, ECG and EtCO2 monitors were checked and applied to each patient on arrival to the operating room. All the patients were allocated into two groups of 30 each. Group 1 (Magnesium): MgSO4 infusion was administered both pre-operatively and intraoperatively. Group 2 (Control): Normal saline was administered both pre-operatively and intraoperatively. 30 mins before induction of anaesthesia, In Group 1, MgSO4 infusion was administered at the rate of 40mg/kg in 100 ml NS IV over 15 mins and same volume of normal saline was administered in group 2. Injection glycopyrrolate (0.004 mg/kg body weight) IV, injection fentanyl (1 µg/kg body weight) IV, injection ondansetron (0.1mg/kg body weight) IV, injection midazolam (0.02mg/kg body weight) IV were given as pre-medication. Hemodynamic parameters (HR, SBP, DBP, and MAP) were recorded before induction, before intubation, after intubation and every 15mins during the intraoperative period till the end of surgery. In group 1, IV infusion of MgSO4 was administered at the rate of 10mg/kg/hr till the end of surgery. In group 2, IV infusion of same volume of isotonic NS was given. GA was induced with injection propofol (2mg/kg body weight) IV, intubated with appropriate sized cuffed endotracheal tube after administration of injection atracurium (0.5mg/kg body weight) IV. Anaesthesia was maintained with O2:N2O in the ratio of 40:60 and incremental doses of injection atracurium IV. At the end of surgery, neuromuscular blockade was reversed with injection neostigmine (0.05mg/kg body weight) IV and injection glycopyrrolate (0.01mg/kg body weight) IV. After recovery from anaesthesia, sedation level was assessed using four point rating scale10. In the recovery room, patient was kept for 4hrs for assessment of analgesia using VAS score and measurement of hemodynamic parameters every hourly. Level of Pain at 0,1,2,3,4,8,16 and 24 hours postoperatively was assessed by using VAS scoring. When VAS ≥ 3, rescue analgesia was provided in the form of injection tramadol (2mg/kg body weight) IV. Hemodynamic parameters i.e., HR, SBP, DBP, MAP were recorded at 0,1,2,3,4, 8,16 and 24 hours postoperatively. Sedation scores: Patient fully awake- Grade-1; Patient somnolent but responds to verbal commands –Grade-2; Patient somnolent but responds to tactile stimulation- Grade-3 and Patient asleep but responds to pain- Grade-4.
Figure 1: Visual Analog Scale
Statistics: In the present study, we used student’s unpaired t-test for statistical analysis. It was used because two sets of population were compared which were independent and identically distributed. ‘p’value: It indicates the probability of error and a value less than 0.05 is considered statistically significant.
OBSERVATIONS AND RESULTS Age wise distribution was almost similar in both the groups.
Table 2: Comparison of demographics in both the groups (n=60)
p-value <0.05 was taken as significant
Table 2: Comparison of age wise distribution in both the groups
Table 3: Comparision of pain score (mean±sd vas) at different intervals in both the groups (n = 60)
p value <0.05 was taken as significant. VAS scores at different intervals were significantly lower in group 1 (magnesium group) except at emergence from anaesthesia (p>0.05).
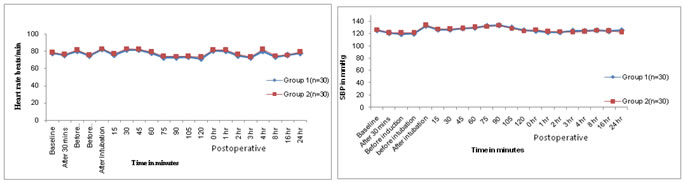
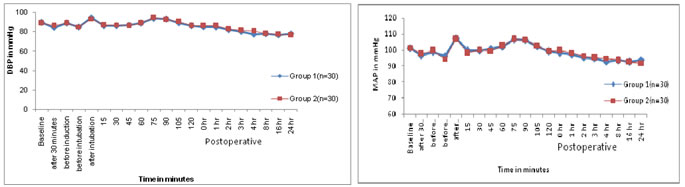
Figure 1: Comparison of heart rates in both the groups (n=60) Figure 2: Comparison of systolic blood pressures in both the groups (n=60) Figure 3: Comparison of diastolic blood pressures in both the Figure 4: Comparison of mean arterial pressures in both the groups (n=60) groups (n=60) Mean of mean arterial blood pressures were comparable between both the groups. In the present study, mean age in the Mg and control groups was 37.06±6.74 and 38.06±6.76 years respectively (Table No.1,2). Mean age among the groups was comparable. The mean age of patients in Mg and control groups of the present study was in accordance with those of S.Kaur et al 4 (2012) (35.26±2.12 and 35.18±2.23), Maana EM et al 5 (2012) (39.55±12.23 and 35.5±9.06) and Shariat Moharari et al 6 (2013) (41.33±10.06 and 45.13±11.74) respectively. In the present study, mean weight in the Mg and control groups was 54.63±5.86 and 55.63±6.16 kgs respectively (Table no. 1). The mean weight of the patients in Mg and control groups of present study was in comparable with those of S. Kaur et al 4 (2012) (49.50±4.33 and 51.04±5.17) respectively. The mean weight of the patients in Mg and control groups in the study conducted by Maana EM et al5 (2012) was (74.65±8.02 and 72.50±7.97) and Shariat Moharari et al (2013) (69.36±11.41 and 67.94±6.36) respectively. In the present study, total male patients in Mg and control groups were 18 and 16 respectively, while female patients in Mg and control groups were 12 and 14 respectively (Table no.1). Male to female ratio of Mg and control group in present study was in accordance with the studies of S.Kaur et al 4 (2012), Maana EM et al 5 (2012), Shariat Moharari et al (2013) respectively. In the present study, mean sedation score at recovery in the Mg and control groups was 1.93±0.63 and 1.56±0.56 respectively (Table No.1). Mean sedation score at recovery in Mg group was higher than in control group which was statistically significant (p<0.05). The mean sedation score of the patients in Mg and control groups of present study was in accordance with those of Kiran S et al3 (2011) (1.86±0.64 and 1.40±0.49) respectively. S Kaur et al 4 (2012) stated that Mean sedation score was statistically higher in Mg group patients than in control group patients. Thus, mean sedation score at recovery was higher in Mg group as compared to control group as Mg is considered as central nervous system depressant. In present study, mean VAS scores at different intervals in magnesium group were lesser than in the control group which was statistically significant (p <0.05). But, at emergence from anaesthesia, mean VAS score was statistically insignificant (Table No. 1). The mean VAS score in the present study was in accordance with the VAS scores in Kiran S et al 3 (2011), Mohammed Shawagfeh et al 7 (2012) and S Kaur et al 4 (2012) at different intervals post-operatively. Shariat Moharari R et al 6 (2014) stated that postoperative pain was significantly lower during the first 24 hour in Mg group. Manna EM et al 5 (2012) reported that postoperative pain assessments by VAS showed significantly lower pain scores in Mg group compared to control group. Koinig et al8 (1998) stated that the mean intraoperative and postoperative fentanyl consumption in the control group was significantly higher in control group than that in the Mg group thus concluding that preoperative administration of magnesium reduces not only postoperative, but also intraoperative, analgesic requirements and thus magnesium can be be an adjuvant to perioperative analgesic management. Seyhan et al 9 (2006) stated that magnesium groups had consumed significantly less morphine than control group during the study period. Thus, they concluded that perioperative administration of magnesium significantly reduces analgesic consumption intraoperatively and requirement of analgesia on demand postoperatively. Tramer MR et al50 observed that pretreatment with IV magnesium sulphate had no impact on postoperative pain and analgesic consumption, but the patients in their study received only diclofenac suppository immediate preoperatively. Thus, it was seen in the present study that mean VAS scores were lesser in Mg group as compared to that in control group. The number of mean rescue analgesics required postoperatively was lesser in Mg group (1.23) compared to that in control group (2.43). Kiran S et al3 (2007) have stated that rescue analgesic requirement of patients in Mg group was lesser than that in the control group. Ryu JH et al10 (2008) have observed that cumulative postoperative analgesic consumption was statistically lesser in Mg group compared to that in control group. Kaur S et al4 (2012) have stated that the cumulative analgesic requirement in 24hrs was statistically significantly lesser in Mg group compared to that in control group. Thus, the total requirement for analgesics 24 hours postoperatively was lesser in magnesium group compared to the requirement in the control group, thus concluding the effect of magnesium sulphate an an adjuvant analgesic which reduces the requirement of analgesics.
DISCUSSION Uncontrolled post operative pain may produce a range of detrimental acute and chronic effects. The attenuation of perioperative pathophysiology that occurs during surgery through reduction of nociceptive input to the central nervous system and optimization of perioperative analgesia may decrease complications and facilitate recovery during the immediate post-operative period11. Transmission of nociceptive stimuli from the periphery to the central nervous system results in neuroendocrine stress response involving hypothalamic pituitary adrenocortical and sympathoadrenal interactions. This results in sodium and water retention, increased levels of blood glucose, free fatty acids, ketone bodies and lactate. A hypermetabolic state occurs, oxygen consumption increases and metabolic substrates are mobilized from storage depots. Increased level of blood glucose may cause poor wound healing and depression of immune function. The negative nitrogen balance and protein catabolism may impede convalescence. The stress response may be an important factor in the post operative development of hypercoagulability.11 Sympathetic activation may increase myocardial oxygen consumption which may be important in the development of myocardial ischemia and infarction and may also delay the return of postoperative gastrointestinal motility which may develop into paralytic ileus. Patients with poor pain control may breath less deeply, have inadequate cough and are susceptible to the development of post operative pulmonary complications. Thus, attenuation of the stress response and post operative pain may facilitate and accelerate the patient’s recovery post operatively. In the present study, pain management was started prior to pain initiation on the basis of preemptive analgesia. The aim of preemptive analgesia, which has been investigated in recent years, is to provide analgesia prior to a painful stimulus to prevent central sensitization caused by the painful stimulus such as tissue injury during surgery13, in an attempt to obtain better pain relief compared with when the same analgesic intervention is used after the painful stimulus is given. Consequently, immediate postoperative pain may be reduced and the development of chronic pain may be prevented. Most commonly used IV agents as preemptive analgesics are NSAIDs, opioids and NMDA receptor antagonists. Recently, the importance of Mg in anaesthetic practice has been highlighted.14 Mg is a non-competitive NMDA receptor antagonist and a calcium channel blocker with antinociceptive effects. It has been suggested that Mg has the potential to treat and prevent pain by acting as an antagonist of NMDA receptors15,16. No adverse effects of Mg were seen in the study dosage as IV MgSO4 infusion is considered to be safe17. Therefore, it may be worthwhile to use MgSO4 for supplementation to intraoperative anaesthetics and postoperative analgesia, since this molecule is inexpensive, relatively harmless, and the biological basis for its potential antinociceptive effect is promising. Hence, the present study was undertaken to evaluate the effectiveness of IV MgSO4 as a preemptive analgesic in surgeries done under GA and to assess its effects on postoperative pain scores. The concept of preemptive analgesia to reduce the magnitude and duration of postoperative pain was paved in 1983 by Woolf who showed evidence for a central component of post injury pain hypersensitivity in experimental studies18. Therapies that have been tested in preemptive trials include parenteral and oral NSAIDs, sublingual and IV opioids, LA, GABA analogues, parenteral NMDA receptor antagonists.19 However each group of drugs has its own limitations. Use of parenteral NMDA receptor antagonists for providing preemptive analgesia triggered their use. NSAIDs have long been used for preemptive, intraoperative and postoperative analgesia. NSAIDs exert anti inflammatory and analgesic effects through inhibition of prostaglandin synthesis by blocking the activity of cyclooxygenase (COX) but they also inhibit platelet function, increase perioperative bleeding, cause gastrointestinal ulceration and have nephrotoxic effects in patients with and without pre-existing renal insufficiency20. Opioids through their interaction with various opioid receptors produce profound and prolonged analgesia but their clinical use is restricted owing to their potential side effects such as nausea, vomiting, urinary retention, sedation, ileus and respiratory depression. LA are membrane stabilizing drugs which act mainly by inhibiting sodium influx through voltage gated sodium channels, hence inhibiting the generation of action potential. They are used in neuraxial blocks, peripheral nerve blocks, and wound infiltration, often in combination with other drugs mainly opioids. Analgesic action of systemic antiepileptics (GABA analogues) such as pregabalin and its developmental precursor gabapentin are mediated through their binding to the alpha-2 delta subunit of voltage gated calcium channels. Though gabapentin and pregabalin have established their role in effectively reducing the perioperative pain intensity, producing very few adverse effects, further studies are needed to determine the long-term benefits, optimal dose, and duration of treatment of these two drugs21. Magnesium, the fourth most common cation in the body, has numerous physiological activities, including activation of many enzymes involved in energy metabolism and protein synthesis22. It has also been widely used as a tocolytic agent and an anticonvulsant for the treatment of preterm labour23 and preeclampsia24 respectively. On the other hand, Mg is a non-competitive NMDA receptor antagonist with antinociceptive effects25, shortens anaesthetic induction by propofol26 and reduces anaesthetic requirements. When MgSO4 was added to LA for spinal anaesthesia, the duration of anaesthesia was prolonged, postoperative analgesic requirement was reduced and the side effects of high doses of LA and opioids were decreased. Mg has been examined viadifferent routes of administration (systemic, topical, intrathecal, and epidural) by several investigators for the prevention of postoperative pain. Among the routes of administration, the systemic route is the most studied and likely to have a greater level of therapeutic adherence by perioperative clinicians. Thus, by acting as NMDA receptor antagonist and calcium channel blocker, MgSO4 prevents induction of central sensitization due to peripheral nociceptive stimulation and abolish the hypersensitivity once it is established. These characteristics of Mg (anaesthetic and analgesic sparing effect) enable anaesthesiologists to reduce the use of anaesthetics during surgery and the use of analgesics after surgery. Therefore, present study was designed to evaluate the analgesic efficacy of intravenous magnesium sulphate during post operative period after surgeries done under general anaesthesia. In the present study, the changes in mean values of HR in both the groups after administration of study drug were not statistically significant. In both groups there was an initial slight rise in blood pressure which was in accordance with the expected stress response to tracheal intubation during administration of GA. The findings of present study were in accordance to the study conducted by S Kaur et al4 (2012) which stated that intraoperative and postoperative haemodynamic parameters were very much comparable between the two groups (P>0.05). Kiran S et al3 (2004) and Mohammed Shawagfeh7 (2012) stated that comparison of haemodynamic paremeters during study medication and intraoperative period between the two group at different time intervals was statistically insignificant. Koinig et al 8 (1998) stated that hemodynamic and respiratory variables, such as DBP,SBP,HR,SpO2 were similar in both groups and that there were no cases of intra- or postoperative hemodynamic instability during the observation period.In the present study, postoperative hemodynamic parameters (SBP,DBP,MAP,SpO2) were comparable between the two groups. Continuous ECG monitoring did not show any dysrthymias or ischaemic changes. RR, SpO2,EtCO2 throughout the study did not show any abnormalities. No adverse effects like bradycardia, hypotension, PONV, dysrthymias etc were observed during the study. The present study, was undertaken at Kamineni Institute of Medical Sciences Narketpally, Nalgonda to evaluate the efficacy of intravenous magnesium sulphate on postoperative analgesia after operations done under general anaesthesia, during the period September 2012 and October 2014. Sixty patients of ASA grade I and II of 20 to 50 years age, undergoing surgeries under general anaesthesia were divided into two groups of 30 each. Group 1 received 40mg/kg of intravenous infusion of magnesium sulphate preoperatively and 10mg/kg/hr intraoperatively and group 2 received same volume of normal saline preoperatively and intraoperatively. Observations were tabulated and analysed using ‘students unpaired t-test’. The demographic profile (age, age wise distribution, gender wise distribution, weight, weight wise distribution) was comparable in both the groups. Haemodynamic parameters (heart rate, systolic, diastolic blood pressure and mean arterial pressure) were comparable in both the groups. The mean VAS score postoperatively in magnesium group at 0,1,2,3,4,8,16,24 hours was 1.96±0.66, 1.46±0.50, 1.20±0.48, 1.33±0.47, 1.43±0.62, 3.00±0.45, 1.70±0.53, 0.86±0.34 respectively. The mean VAS score postoperatively in control group at 0,1,2,3,4,8,16,24 hours 2.56±0.56, 1.96±0.41, 1.76±0.56, 1.96±0.41, 2.30±0.53, 4.16±0.69, 2.36±0.61, 2.00±0.26 respectively. The decrease in pain scores in magnesium group was statistically significant (p<0.05). The mean number of rescue analgesics required postoperatively is lesser in magnesium group compared to that in control group. The mean sedation score at recovery was 1.93±0.63 in magnesium group and 1.56±0.56 in control group. The higher level of sedation in magnesium group at recovery was statistically significant (p<0.05). No adverse effects like bradycardia, hypotension, dysrythmias were observed.
CONCLUSION From the present study it is concluded that IV administration of MgSO4 in the dosage of 40mg/kg preoperatively and 10mg/kg/hr intraoperative for surgeries done under GA has the following advantages. It is an adjuvant preemptive analgesic, significantly reduces postoperative pain and analgesics requirement. It produces sedation in which patients were asleep and easily arousable. It is haemodynamically stable. It was not associated with any adverse effects and hence can be an attractive alternative for other analgesics like opioids, NSAIDs etc. However, study with larger samples are required to confirm the above findings.
REFERENCES
|
|
 Home
Home