Official Journals By StatPerson Publication
|
Table of Content - Volume 3 Issue 1 -July2017
A comparative study of dexmedetomidine and nalbuphine as an adjuvant to bupivacaine in lower limb surgeries done under epidural anaesthesia
Sonali M Khobragade1*, Jagdish Kalbhor2, Ruchi Saran3, Sandhya Manjrekar4, Soma Cham5
1Associate Professor, 2Resident, 3Senior Resident, 4Professor and HOD, Department of Anaesthesiology, Indira Gandhi Government Medical College and Hospital, Nagpur, Maharashtra, INDIA. 5Associate professor, Dept. of Anaesthesiology, Government Medical College And Hospital, Gondia, Maharashtra, INDIA. Email: drsonalibhagat@yahoo.in
Abstract Aims and Objectives: To compare the clinical profile of Nalbuphine and Dexmedetomidine as an adjuvant to Bupivacaine when administered epidurally with respect to sensory and motor blockade, level of sedation and duration of analgesia. Material and Methods: A study was carried out in the Department of Anaesthesiology of Indira Gandhi Government Medical College and Hospital, Nagpur from Nov 2014 to October 2016. The study included 70 adult patients undergoing infraumbilical and lower limb surgeries in epidural anaesthesia. Assessment of motor blockade was done with Modified Bromage Scale. Degree of pain was evaluated by Visual Analogue Scale. Level of Sedation was assessed by Five Point Scale. Result: There was statistically significant difference between the groups for onset of sensory block, duration of analgesia and reversal of motor blockade. The onset of motor blockade, time to achieve maximum sensory and motor blockade, sedation score, Visual analogue scale score were comparable between the groups. Conclusion: Dexmedetomidine is a better adjuvant to Bupivacaine for epidural anaesthesia when compared to Nalbuphine as it provides earlier onset of sensory blockade, prolonged duration of sensory block and postoperative analgesia with stable vitals and minimal side effects. Key Words: Dexmedetomidine, Nalbuphine, Bupivacaine, Epidural anaesthesia, Infraumbilical surgeries.
INTRODUCTION Epidural anaesthesia is an effective technique used both for providing anaesthesia and postoperative analgesia. It causes intraoperative hemodynamic stability with less stress response causing reduced complications and better patient outcome. It helps in early mobilization by effectively relieving postoperative pain leading to decreased incidence of thromboembolic events. Bupivacaine is a long acting, effective local anaesthetic, commonly administered by the epidural route for surgical anaesthesia as well as for the relief of postoperative pain. Despite its undoubted efficacy, it is associated with cardiotoxicity and neurotoxicity at higher doses1. Adjuvants are being added to local anaesthetics to increase the quality of intraoperative anaesthesia and also attribute to a prolonged and better anti-nociceptive action in the immediate post-operative period. Additions of adjuvants have also contributed in reducing the doses of local anaesthetics and thus reduce the incidence of local anaesthetics toxicity. Various adjuvants like Opioids (Morphine2, Buprenorphine3, Fentanyl4, Pethidine5, Tramadol6, Nalbuphine7 Ketamine8, Midazolam9, Alpha-2 adrenergic agonist (Clonidine10, Dexmedetomidine10) have been used along with Bupivacaine with varied effects. Dexmedetomidine, made up of medetomidine’s dextrogyrous enantiomer, is currently considered a super selective alpha-2 adrenergic agonists prototype (alpha 1: alpha 2 - 1:1600). It is about 8 times more selective towards alpha-2 adrenoreceptor. It has been found to have analgesic, sedative, anxiolytic, neuroprotective and anaesthetic sparing effect with better hemodynamic stability. It causes more intense motor blockade and co-operative sedation without increasing the incidence of side effects11. Nalbuphine (a derivative of 14-Hydroxymorphine) is an opioid with mixed kappa agonist and mu antagonist properties. Its action on the kappa receptors attributes good sedative properties, whereas, partial agonism at the mu receptors induces a ceiling effect on respiratory depression. It is also known to potentiate the action of local anaesthetics. Administered epidurally, it exerts its action by its interaction with opioid receptor present on the spinal cord7. The anaesthetic and analgesic requirement of local anaesthetics gets reduced by the use of these two adjuvants because of their analgesic properties and augmentation of local anaesthetic effects10. Nalbuphine is a strong analgesic with mixed k agonist and µ antagonist properties,act principally on kappa receptors. The site of action in spinal cord is substansiagelatinos. Dexmedetomidine causes hyperpolarisation of nerve tissues by properties altering the transmembrane potential and ion conductance at locus coeruleus in the brainstem12.
MATERIAL AND METHODS The present study was conducted in the Department of Anaesthesiology at IGGMCH, Nagpur during the period of November 2014 to October 2016. After approval from the Institutional ethics committee, this prospective, observational study was conducted to assess and compare the efficacy and clinical profile of Nalbuphine and Dexmedetomidine as an adjuvant to Bupivacaine in epidural anaesthesia. This study was carried out in 70 adult patients admitted in the department of Orthopaedics, with age in the range of 18-50 years, posted for infraumbilical and lower limb surgeries under epidural anaesthesia. All the patients were included in the study only after obtaining a written informed consent. The Inclusion criteria were
Patients who had contraindications to regional anaesthesia like bleeding diathesis, local infection and patients on anticoagulants, patients with spinal deformities, seizure disorder and neurological disease, cardiovascular diseases like arrhythmias, ischaemic heart disease and valvular heart disease, Liver, Respiratory, Kidney and Endocrine diseases and haemodynamically unstable patients were excluded from the study. Detailed pre-anaesthetic evaluation of the patients was performed by an anaesthesiologist a day before the surgery. Preliminary Investigations in the form of; Complete blood count, Random blood sugar, Bleeding time, Clotting time, Coagulation profile, Liver function tests, Kidney function tests, Electrocardiography (ECG), Chest x ray postero-anterior (PA) view were noted. All patients were kept nil by mouth for 8 hrs. All patients were given overnight sedation in the form of Tab. Diazepam 5 mg orally a day prior to surgery. In operation theatre, multipara monitoring device with ECG, pulse rate, non invasive blood pressure, SPO2 was attached to the patient and baseline parameters were noted. Co-loading with 10 ml/kg body weight of intravenous Ringer lactate was done after establishing intravenous line with 18 G cannula, 10 to 15 minutes before the block. Thereafter, intravenous fluids were calculated and given as per body weight and operative loss. Patients also received Inj. Ranitidine 50 mg and Inj.Ondansetron 4 mg IV slowly as a premedication. Patients were then made to sit up for epidural catheterization. Under all aseptic precautions, skin over the desired site was infiltrated with 2 ml of 2% Lignocaine. Epidural space at L2-L3 or L3-L4 interspaces was located using 18G Tuohy needle with midline approach by using loss of resistance technique. Epidural catheter, 18 gauge, placed at about 4 cm in epidural space. Epidural catheter was fixed aseptically. Patient was then made supine. After exclusion of blood in the epidural catheter with negative aspiration, test dose of 3ml inj. Lignocaine with adrenaline (1:2,00,000) was administered to exclude intrathecal or intravascular placement of the catheter. After 5 minutes of administering test dose, patients were randomly allocated to any of two groups of 35 each. Group N: 15 ml Bupivacaine (0.5%) with Nalbuphine 250 mcg/kg epidurally (diluted with NS to make total volume 17 ml). Group D: 15 ml Bupivacaine (0.5%) with Dexmedetomidine 1mcg/kg epidurally (diluted with NS to make total volume 17 ml). Intra operatively all patients were monitored for: Heart rate (HR),Blood pressure(systolic, diastolic and mean), Respiratory rate, SPO2, Sensory block: Onset and duration, Motor block: Onset and density of block using modified Bromage scale, Maximum sensory and motor blockade achieved, Visual anologue scale score for pain assessment, Sedation score. Intraoperatively and postoperatively, bradycardia (heart rate <60 beats per minute) was to be treated with 0.3mg of injection atropine and hypotension (systolic blood pressure falling more than 20% basal value or less than 80mm Hg ) with 3-6mg injection mephenteramine as a bolus. Respiratory depression (SpO2 < 90% or Respiratory rate < 8 breaths/minute) if any, was to be treated by administration of 100% O2 with face mask or ventilation with IPPV accordingly. Assessment of Sensory block
Assessment of Motor block The time of onset of motor blockade, the degree of motor blockade and duration of motor blockade were recorded. The degree of motor blockade was assessed by modified Bromage scale13. Modified Bromage scale 0= No motor block. 1=Inability to raise extended leg; able to move knees and feet 2=Inability to raise extended leg and move knee; able to move feet 3=complete block of motor limb.
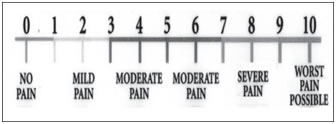
1= alert and wide awake. 2= arousable to verbal commands. 3= arousable to gentle tactile stimulation. 4= arousable to vigorous shaking. 5= unarousable. During surgical procedure and postoperatively adverse effects like anxiety, nausea, vomiting, dry mouth, respiratory depression, pruritis, urinary retension and shivering were recorded and treated accordingly. Anxiety was treated with inj. Midazolam 1mg, nausea and vomiting treated with inj. Ondensetron, pruritis and shivering was treated with inj. Promethazine 12.5mg. Monitoring was continued in the postoperative period every 6 hourly for 24 hours. Visual analogue scale13: Visual analogue scale consists of a 10 cm line, marked at 1 cm each. The patient makes a mark on the line that represents the intensity of pain he or she experiences. Mark “0” represent no pain and mark “10” represents worst possible pain. The numbers marked by the patient was taken as units of pain intensity. 0= no pain 10 maximum pain
Figure 1:
Rescue analgesia was provided with epidural top up by 8 ml of 0.125% Bupivacaine when patient complained of pain for the first time postoperatively with visual analogue score >4. Subsequent epidural top ups were given 8 hourly with 8 ml of 0.125% Bupivacaine. Statistical Analysis Data were collected, tabulated, coded then analyzed using SPSS ® computer software version 20.0.
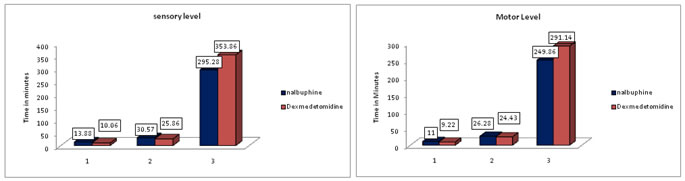
RESULTS 70 patients were enrolled in the study with comparable demographic characteristics such as age, weight, height and duration of surgery. SENSORY BLOCK CHARACTERISTICS (Figure 1): The onset of sensory blockade was achieved early with mean time of 10.06 (±4.42) minutes in group D (Dexmedetomidine) which showed significant difference from group N (Nalbuphine) where the mean time for onset of sensory blockade was 13.88 (±7.83) minutes. (p=0.014). The median maximum sensory level achieved in group N was T6 and median maximum sensory level achieved in group D was T5.The mean(±SD) time to reach maximum sensory level in group N( 30.57 ±13.87 minutes) was comparable with group D (25.86±8.62minutes) (p=0.092). The mean (±SD) time to two segment regression in group N was 93.43 (±20.28) minutes and in group D was 93.71 (±20.16) minutes. The mean (±SD) time for regression of sensory level to T10 in group N was 129.28 (±25.67) minutes and in group D was 131.43 (±24.51) minutes. The mean time for two segment regression and regression of sensory level to T10 were comparable in both the groups. The mean (±SD) duration of analgesia was significantly prolonged in group D, with duration of 353.86 (±51.36) minutes as compared to group N, having mean duration 295.28(±65.95) minutes. (p<0.000).
Figure 2: Showing sensory block characteristics Figure 3: Showing motor block characteristics
1- Sensory onset time. 2- Time to achieve maximum sensory level. 3- Duration of Analgesia MOTOR BLOCK CHARACTERISTICS (FIGURE -2): The mean (SD) time for onset of motor blockade in group N (Nalbuphine) was 11.0 (±6.28) minutes and in group D (Dexmedetomidine) was 9.22 (±3.87) minutes which statistically not significant (p=0.160) (NS). The mean (SD) time to reach maximum motor blockade in group N (Nalbuphine) was 26.28 (±16.24) minutes and in group D (Dexmedetomidine) was 24.43 (±11.74) minutes which was statistically not significant (p=0.58). The mean (SD) time for complete reversal of motor blockade was prolonged in group D (291.14 ±51.59 minutes) than in group N (249.86 ±58.80 minutes) (p<0.003).Hence, the motor blockade recovered earlier in group N.
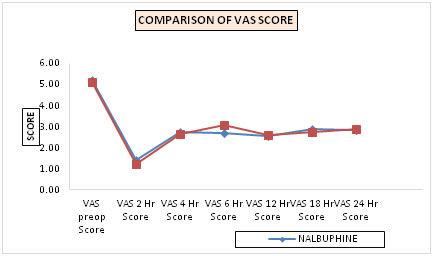
Table 1: Comparison of mean vas scores in the two groups (line diagram - 3)
The pain scores were noted at 0 hr, ½ hr, 1 hr, 1.5 hr, 2 hrs, 4 hrs, 6 hrs, 12 hrs, 18 hrs, 24 hrs postoperatively. The mean (SD) VAS scores in group N and group D were comparable at various time intervals. Figure 3: Line diagram-3 showing mean vas score
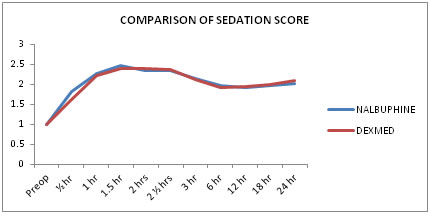
Table 2: Comparison of the mean (±sd) sedation score (line diagram-4)
The mean (±SD) sedation scores in group N and group D were statistically not significant at various time intervals.
Figure 4: Showing mean sedation score
Table 3: Perioperative complication
The hypotension and bradycardia was not observed in any patients of either group. No major differences in haemodynamics were found in both the groups. Nausea was observed in 3 (8.57%) patients and vomiting was observed in 1(2.8%) patient in group N while no patient in group D had nausea and vomiting. Dry mouth was observed in 1 (2.8%) patient in group D.
DISCUSSION Perioperative pain management is one of the important task to the anaesthesiologist. Pain relief is necessary for both humanitarian and therapeutic reasons. Uncontrolled pain in the postoperative period can have detrimental physiological effects. Pain can greatly impede the return of normal pulmonary functions such as inability to cough, bronchospasm which leads to atelectasis and hypoxemia especially in upper abdominal and thoracic surgeries14. Pain promotes immobility and hence increases the risk of deep vein thrombosis. Pain leads to the stress response, increased catecholamine release, increased oxygen demand and increased cardiac work. Increased catabolic response to surgical trauma impairs immune mechanisms and delays wound healing. Hence, adequate pain relief undoubtedly decreases morbidity and mortality. Over the period of years, there is advent of various sophisticated techniques in anaesthesia. Regional anaesthesia has various advantages over general anaesthesia such as reduced incidence of deep vein thrombosis, pulmonary embolism, reduced cardiac complications in high risk patients, reduced bleeding and transfusion requirement. Regional anaesthesia allow earlier return of gastrointestinal function, decreased stress response may result in less perioperative ischemia and reduced morbidity and mortality in patient with coronary artery disease15. Regional anaesthesia may also preserve immunity perioperatively and allow earlier wound healing16. In recent times, the role of epidural and subarachnoid opioids for the relief of post-operative pain promotes a new platform in this field. This is because of the direct action of the opioids on specific opioid receptors that are richly distributed in the posterior horn of the spinal cord. The epidural opioids have a wider margin of safety as against systemic opioids. Though the opioids reduce the toxicity and cardiovascular effects of local anesthetics this type of combinations may bring about additional undesirable problems like itching, nausea and vomiting and / or respiratory depression12. Nalbuphine is a drug with mixed µ antagonist and k agonist properties. Nalbuphine has the potential to maintain or even enhance µ-opioid based analgesia while simultaneously mitigating the µ-opioid related side effects. Nalbuphine and other k agonists have provided potent analgesia in certain models of visceral nociception. They demonstrate complicated interactions with µ opiates that suggest dose-dependent synergies and significant antagonisms at larger doses. Hence, Nalbuphine was considered as an adjuvant drug in terms of its ability to produce an antagonism of the side effects attendant to spinal opiates, e.g. respiratory depression, pruritus and urinary retention12. Alpha-2 adrenergic receptor (AR) agonists have been the focus of interest due to sedative, analgesic, perioperative sympatholytic and hemodynamic stabilizing properties. Alpha2-receptors are found in many sites throughout the body. Alpha2 - adrenoceptors are found in peripheral and central nervous systems, in effector organs such as the liver, kidney, pancreas, eye vascular smooth muscles and platelets. Dexmedetomidine is a new alpha2-agonist approved for use. It has sedative, analgesic, sympatholytic and anxiolytic effect that blunts many cardiovascular responses in the perioperative period. It causes sedation without causing respiratory depression. It is suggested that intrathecal Dexmedetomidine produces its analgesic effect by inhibiting the release of C fibers transmission and by hyperpolarization of post-synaptic dorsal horn neurons12. Taking all this into consideration, this present study was designed to assess and compare the efficacy and clinical profile of Nalbuphine and Dexmedetomidine as an adjuvant to Bupivacaine in epidural anaesthesia. The two groups were comparable with respect to age, weight and height. All the patients in group N and group D were belonging to ASA grade I. Sensory block characteristics: Onset of sensory block: In our study, the mean (SD) time required for onset of sensory block in group N was 13.88(±7.83) minutes and in group D was 10.06( ±4.42) minutes(p=0.014).Onset of sensory block was significantly earlier in group D than group N. Mittal AA, Agarwal A et al (2016)17 studied the role of Fentanyl and Dexmedetomidine as an adjuvant to Ropivacaine in epidural anaesthesia for infra-umblical gynecologic or orthopedic surgeries. The time of onset of sensory blockade at T10 level was (9.375±1.67) minutes in Group RD, 13.45(±1.99) minutes in Group RF and (15.90±1.59) minutes in Group R. Bajwa SJS, Singh A et al (2011)18 studied the hemodynamic, sedative and analgesia potentiating effects of epidurally administered Fentanyl and Dexmedetomidine when combined with Ropivacaine in patients undergoing lower limb surgeries. The onset of sensory analgesia at T10 in the RD group was (7.12±2.44) minutes and in RF group was (9.14±2.94) minutes. The onset of sensory analgesia at T10 in the RD group was significantly earlier than group RF (p<0.05). Karhade SS et al (2015)19 conducted a study to assess the effects of Dexmedetomidine on quality and efficacy of the epidural Bupivacaine (0.5%) for vaginal hysterectomies. The onset of analgesia at T10 dermatomal level in group I (Bupivacaine) was 17.12( ± 2.44) minutes and in group II (Bupivacaine with dexmedetomedine) was 10.14(± 2.94) minutes. The onset of analgesia was significantly earlier in group II as compared to group I (P < 0.001). Michael RM, Mehta M et al (2016)12 studied the effects of Nalbuphine and Dexmedetomidine, used as an adjuvant in spinal anaesthesia for lower abdominal and lower limb surgeries. The onset of sensory blockade in Group D was (3.5±1.106) minutes and in Group N was (6.3±1.64) minutes. The onset of sensory blockade was significantly earlier in group D than group N (p<0.00). Duration of analgesia: In our study, the mean (±SD) duration of analgesia in group N (Nalbuphine) was 295.28(±65.95) minutes and in group D (Dexmedetomidine) was 353.86 (±51.36) minutes (p=0.000)(HS).Hence, the duration of analgesia was significantly prolonged in group D(Dexmedetomidine) than group N(Nalbuphine). Bajwa SJS, Singh A et al(2011)18 found that the duration of analgesia was significantly prolonged in Dexmedetomidine group (366.62±24.42minutes) than Fentanyl group (242.16±23.86 minutes). Michael RM, Mehta M et al (2016)12 compared intrathecal Nalbuphine and Dexmedetomidine for lower abdominal and lower limb surgeries. The duration of analgesia was prolonged in group D (276.07±31.28 minutes) as compared to group N (200.67±22.18 minutes). Ahluwalia P, Ahluwalia A, et al (2015)20 studied the effects of intrathecal Nalbuphine in patients undergoing lower abdominal surgeries. The duration of analgesia was prolonged in group BN (298.43±30.92 minutes) as compared to group B (178.67±28.34 minutes).These findings from the studies mentioned above concur with the finding from our study regarding onset of sensory block and duration of analgesia. Motor block characteristics: Onset of motor blockade: In our study, the mean (SD) time for onset of motor blockade in group N (Nalbuphine) was 11.0 (±6.28) minutes and in group D (Dexmedetomidine) was 9.22 (±3.87) minutes (p=0.160)(NS). The time for onset of motor blockade was comparable in both the groups. Michael RM, Mehta M et al (2016)12 observed the earlier onset of motor blockade in Dexmedetomidine group (6.23±1.38 minutes) than Nalbuphine group (8.73±1.87 minutes) when compared intrathecal Nalbuphine and Dexmedetomidine. Time to achieve maximum motor blockade: In our study, the mean (SD) time to reach maximum motor blockade in group N (Nalbuphine) was 26.28 (±16.24) minutes and in group D (Dexmedetomidine) was 24.43(±11.74) minutes. The time to reach maximum motor blockade was comparable between the groups. (p=0.585)(NS). Attri JP, Kaur S et al (2014)21 found that the mean (SD) time to reach maximum motor blockade in Dexmedetomidine group was 25.73(±4.172) minutes and in Ropivacaine group was 27.34(±5.97) minutes. The difference between the time to reach maximum motor blockade in two groups was statistically not significant. Mittal A A, Agarwal Aet al (2016)17 observed that the mean (SD) time to reach maximum motor blockade in group RD was 25.45(±3.36) minutes, in group RF was 36.50(±4.96) minutes and group R was 45.50(±5.75) minutes. Duration of complete reversal of motor blockade: In our study, the mean (SD) time for complete reversal of motor blockade in group N (Nalbuphine) was 249.86(±58.80) minutes and in group D (Dexmedetomidine) was 291.14 (±51.59) minutes (p=0.003). The complete reversal of motor blockade was significantly delayed in group D than group N. Bajwa SJS, Singh A et al(2011)18 found that the mean (SD) duration of complete motor recovery was delayed in group RD (259.62 ±21.38 minutes) than in group RF was (178.52 ±23.29 minutes). Ahluwalia P, Ahluwalia A et al (2015)20observed delayed motor recovery in group BN (256.41±33.41 minutes) than group B (178.67±28.34 minutes) when compared intrathecal Nalbuphine with Bupivacaine and Bupivacaine alone. Karhade SS, Acharya SA et al (2015)19 observed that the duration of complete motor recovery was delayed in Dexmedetomidine group (320.62±23.86 minutes) than Bupivacaine group (150.24±24.42 minutes). Michael RM, Mehta M et al (2016)12 when compared intrathecal Nalbuphine and Dexmedetomidine, also observed that, the duration of complete motor recovery was delayed in group D (247.43±28.53 minutes) than group N (184.17±27.0minutes). The findings of our study regarding motor block characteristics were corresponding to the above mentioned studies. Complications: In our study, 3(8.57%) patients had nausea and 1(2.8%) patient had vomiting in group N while no patient in group D had nausea and vomiting. Dry mouth was observed in 1(2.8%) patient in group D. Attri JP et al(2014)21 noted that 2% of the patients in Bupivacaine group had nausea while 6% in Dexmedetomidine group had nausea. The difference was statistically not significant. Ahluwalia P, Ahluwalia A et al (2015)20studied effect of intrathecal Nalbuphine and noted that nausea was noted in 2 patients in group B and 5 patients in group BN. Bajwa SJS, Bajwa SK et al (2011)10 observed dry mouth in 6(26%) of patients in group RD and 7(28%) of patients in group RC. 4(16%)patients in group RD and 3(12%) patients in group RC had nausea. 1(4%) patient in both groups had vomiting. Shivering was noted in 1(4%) patient in group RD and 2(8%) patients in group RC. 3(12%) patients in group RD and 2(8%) patients in group RC had Dizziness. In our study, Incidence of adverse effects were minimal and managed accordingly.
CONCLUSION From the present study, we conclude that Dexmedetomidine is a better adjuvant to Bupivacaine for epidural anaesthesia when compared to Nalbuphine as it provides earlier onset of sensory blockade, prolonged duration of sensory block and postoperative analgesia with stable vitals and minimal side effects.
REFERENCES
|
|
 Home
Home