Official Journals By StatPerson Publication
|
Table of Content Volume 6 Issue 1 - April 2018
Effect of spinal anaesthesia on perioperative hyperglycemia in diabetic patients undergoing lower limb orthopaedic surgeries
Shaily Gokhru1, Shalini Jain2*
1Sr. Resident, 2Associate Professor, Department of Anaesthesiology, MGM Medical College, Indore, Madhya Pradesh, INDIA. Email: drshalininjain@gmail.com
INTRODUCTION Surgery is considered to be the combination of numerous factors including anaesthesia, medication, tissue trauma, blood loss, and temperature changes. All these factors cause metabolic changes. Together, they produce perioperative adaptive stress response1-3. Afferent pathway of this stress response is formed by the peripheral and central nervous system and local tissue factors, including products of the immunological system. Sympathetic nervous system and hypothalamic- pituitary- adrenal axis constitute the efferent pathway of the perioperative stress response. Surgical tissue trauma and stress results in activation of hypothalamic-pituitary-adrenal axis, thereby causing release of corticotrophin releasing hormone (CRH) by hypothalamus. CRH stimulates release of adreno-corticotropic hormone (ACTH) by anterior pituitary gland. ACTH stimulation induces the secretion of cortisol. Glucocorticoids exhibit complex metabolic effects. They promote proteolysis, glycogenolysis and gluconeogenesis. Cortisol also leads to enhanced lipolysis, which increases the production of gluconeogenic precursors from the breakdown of triglyceride into glycerol and fatty acids. Insulin resistance develops dose-dependently in diabetic and nondiabetic individuals due to increase in free fatty acid levels. This is an important factor in the development of stress hyperglycemia. Furthermore, glucocorticoids produce permissive effect by facilitating effects of catecholamines4,5. Surgical stress also causes hypothalamic activation of the sympathetic nervous system. This in turn results in increased secretion of catecholamines from the adrenal medulla and release of norepinephrine from presynaptic nerve terminals. High catecholamine levels have catabolic effect. They inhibit insulin release and also enhance glycogenolysis, hepatic glucose production and peripheral insulin resistance, producing hyperglycemia[6]. Activation of immune system results in release of cytokines like chemokines, interferons, interleukins, lymphokines and tumour necrosis factor [7,8]. Short term stress response is vital as it provides substrates needed to sustain increased metabolic demands. However, a prolonged, high magnitude stress response has harmful effects on metabolism and immune function. High levels of glucocorticoids, catecholamines and cytokines attenuate protein anabolism, wound healing and the activity of the immune defense system after surgery by causing hyperglycemia, thereby increasing perioperative morbidity and mortality9-12. There are three main methods for attenuating surgical stress response including neural blockade by epidural or spinal anesthesia, which prevent nociceptive signals from the surgical area from reaching the central nervous system. This inhibitor effect involves both afferent and efferent pathways. Cortisol response is suppressed by neural blockade from T4 to S5. Other methods are intravenous administration of high-dose of strong opioid analgesics which block hypothalamic pituitary gland function and infusion of anabolic hormones such as insulin that causes change in the hormonal status of the patient13-15.
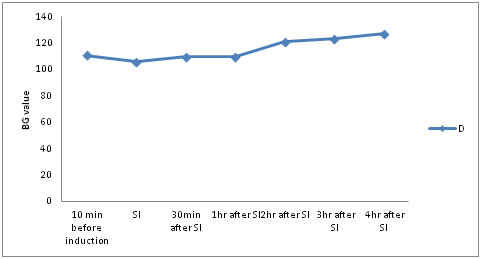
MATERIAL AND METHODS After obtaining approval from the Hospital Ethics Committee and written informed consent from the patients, 60 patients having either Type I or Type II Diabetes Mellitus controlled on either oral hypoglycaemic drugs or injectable insulin aged 35 to 65 years, belonging to either sex and American Society of Anesthesiologists (ASA) physical status IIand III undergoing elective lower limb orthopaedic surgeries under spinal anaesthesia were included in this prospective study. Only patients having preoperative blood glucose level between 80-120mg/dl were included in the study. Patients on recent intravenous or oral steroid therapy within 30 days, although inhaled steroids were permitted, known case of chronic obstructive respiratory disease and asthma on intravenous steroid therapy; having coagulation abnormalities, hypovolemia or hypotension, pre-existing severe bradycardia, or ejection fraction <30%, arrhythmias on ECG or cardiac block; known cases of renal impairment, pregnant or lactating female patients and patients allergic to any drug to be used were excluded from the study. All the patients underwent thorough preanaesthetic evaluation on the day prior to surgery. They were informed about glucose monitoring by glucometer using capillary blood that will be done in perioperative period. Patients were reassured to alleviate their anxieties. All the patients were kept fasting overnight. All patients were shifted on insulin preoperatively. Oral hypoglycaemic drugs were stopped 24 hrs prior to surgery. Basic laboratory investigations were conducted including haemogram, urine analysis, chest x-ray, electrocardiogram, blood sugar, serum creatinine, blood urea, serum electrolytes and coagulation profile. Intravenous access was secured with 18 G cannula. The following monitors were connected to the patients in the operating room- pulse oximeter, non invasive blood pressure monitor and three lead ECG monitoring. All patients received inj. Glycopyrrolate 0.2mg i.m. and inj. Ondansetron 4mg i.v. and inj Midazolam 1mg half an hour before surgery. All the patients were preloaded with 10 ml/kg of 0.9% normal saline. Under aseptic precautions, 3.5ml of 0.5% bupivacaine (heavy) was injected in subarachnoid space via 25G Quincke’s spinal needle at L3-L4 interspace in sitting position. After achieving neuraxial blockade (lack of sensory perception of needle tip sharpness till desired dermatome level) surgery was started. During surgery, pulse rate, non invasive blood pressure and peripheral oxygen saturation were monitored every 15 minute till the completion of procedure. Capillary blood sugar was measured using glucometer under full aseptic conditions at 10 minutes before initiation of anaesthesia, at time of surgical incision (SI), 30 min after incision and thereafter 1hourly till 4th hour after SI. Any episode of bradycardia (HR <60/min or a fall of >20% from basal HR) and hypotension (mean atrial pressure <60 mm Hg or a fall of >20% from basal BP) were recorded and managed as per the standard protocols. When blood glucose concentrations exceeded 180mg/dL, it was treated as hyperglycaemia as per continuous insulin infusion (CII) protocol. When blood glucose concentrations lowered below 60mg/dl, it was treated as hypoglycaemia as per the standard protocol. The sample size was calculated based on previous studies. Statistical testing was conducted with the statistical package for the social science system version (SPSS Statistics for Windows, Version 17.0. Chicago: SPSS Inc.,). Ages, weight, height, duration of anaesthesia and blood glucose (BG) values were reported as mean ± standard deviation. Comparison of BG before, during and after surgery was done using Student's t-test. For statistical test, P < 0.05 was taken to indicate a significant difference. A total of 60 patients were included in the study. Table 1 showed demographic characteristics (age, weight, and height) and duration of anaesthesia. Mean BG value preoperatively or 10min before induction was 110.60±12.082. Then at SI, there was statistically significant decrease in BG value to mean value 105.87±14.583.30min after SI, mean BG value was 109.70±18.958. This value was lower as compared to the pre-operative BG value, but not statistically significant. 1hr after SI, BG value was 109.67±14.221. This value was also lower as compared to the pre-operative BG value, but not statistically significant. 2hrs after SI, BG value increased to mean value 120.83±19.449. There was statistically significant difference(p=.000). Even, 3hrs after SI, BG value continued increasing and the mean value became 123.10±16.003. There was statistically significant difference (p=.000). 4hrs after SI, BG value was maximum with the value being 127.23±16.397. There was statistically significant difference (p=.000). Blood glucose (BG) value decreases till 1hr after surgical incision (SI), and then increases till 4th hr after SI. This change in blood glucose values is statistically significant at SI, 2nd hr after SI, 3rd hr after SI and 4th hr after SI. (Table 2 and 3)
Table 1: The demographic characteristics (age, weight, and height) and duration of anaesthesia
Table 2: Mean and SD of blood glucose (BG) values (mg/dl)
Table 3: Trend of blood glucose (BG) values taking BG value at 10min before induction as reference value(in mg/dl)
Positive mean values indicate that they are lower than BG value at 10min before induction. Negative mean values indicate that they are higher than BG value at 10min before induction. Figure 1. Trend of blood glucose (BG) values taking BG value at 10min before induction as reference value (in mg/dl) Figure 1:
DISCUSSION The stress of surgery results in increased levels of gluco-regulatory hormones (catecholamines, cortisol, glucagon, and growth hormone) and excessive release of inflammatory cytokines, such as tumor necrosis factor, interleukin-6 and interleukin-1). The counter-regulatory response produces alterations in carbohydrate metabolism, including insulin resistance, increased hepatic glucose production, impaired peripheral glucose utilization, and relative insulin deficiency9-10. Increase in cortisol levels occurs at the start of surgery due to ACTH stimulation. This change may even occur fourfold during the first 4–6 h, depending on the extent of the surgery. The cortisol response also varies according to the anesthetic approach. ACTH secretion is generally inhibited by increased cortisol. However, failure of this mechanism occurs after surgery, and hormone values remain high. Cortisol has a complicated effect on carbohydrate, protein, and fat metabolism, causing gluconeogenesis, proteolysis and lipolysis in the liver. During anesthesia induction and surgery, insulin concentration may decrease due to α adrenergic inhibition of β-cell secretion. Plasma glucose concentrations increase in perioperative period. In fact, anaesthesia itself results in hyperglycemia, which is then further aggravated by the surgical procedure. The initial increase in plasma glucose after injury is due to activation of glycogenolysis. But later hepatic gluconeogenesis becomes the major factor in liver glucose release because liver glycogen stores are limited. The usual mechanisms that maintain glucose homeostasis are ineffective in the perioperative period and catabolic hormones promote the production of glucose, thereby resulting in hyperglycemia1-3,11-13. Spinal anaesthesia inhibits transmission of impulses from the site of trauma by producing neural blockade. Regional anesthesia has a direct effect on the hyperglycemic response to surgery which depends on the secretion of stress hormones, and is mediated by afferent and efferent neural pathways. Cortisol response is suppressed by neural blockade from T4 to S5 dermatomal level13-15. Poon et al. achieved better stress response control by combining epidural anesthesia with general anesthesia16. Opioids also suppress the stress response by inhibiting hypothalamic pituitary gland function. In a study of lower abdominal surgery, 50 µg/kg fentanyl suppressed the stress response by reducing growth hormone, cortisol, and glucose concentrations. But, systemic opioids may be insufficient to suppress this response in upper abdominal surgeries. In other study of cholecystectomies using 100 µg/kg fentanyl, the stress response was suppressed; however, patients also required postoperative ventilator support. Most studies of neural blocks have assessed the effect of epidural anesthesia, but, few have addressed spinal anesthesia and stress. Moller et al. (1984) compared stress responses following spinal and general anesthesia in abdominal hysterectomies, and reported that spinal anesthesia had a temporary inhibitory effect, which was correlated with the sensorial block level14. Cigdem YILDRIM GUCLU et al.(2013) compared the neuroendocrine and hemodynamic effects of general and spinal anaesthesia for minimally invasive lumbar disc surgery. There were significant differences in cortisol values at 30 min after surgery (p=0.00). Results showed that spinal anesthesia can be a good alternative to general anesthesia for single-level lumbar disc surgery15. According to Basem et al. (2013) for all patients combined, mean glucose increased slightly from preoperative to incision, substantially from incision to surgery midpoint, and then remained high and fairly stable through emergence, with nondiabetic patients showing a greater increase. For nondiabetics, the mean increase in glucose concentration was more in patients given dexamethasone than placebo. However, there was no dexamethasone effect in diabetics. They assessed this response in patients undergoing non-cardiac surgery under general anaesthesia17. The aim of our study was to assess the effect of spinal anaesthesia on perioperative hyperglycemia in diabetic patients undergoing lower limb orthopaedic surgeries and also to state the trend of perioperative hyperglycemia. In diabetic (D) group, blood glucose (BG) value decreases till 1hr after surgical incision (SI), and then increases till 4th hr after SI. This change in blood glucose values is statistically significant at SI, 2nd hr after SI, 3rd hr after SI and 4th hr after SI. The effect of spinal anaesthesia on perioperative hyperglycemia in diabetic patients is not well established. In the presence of an absolute or relative deficiency of insulin, increased catecholamines and glucagon levels lead to increased gluconeogenesis and glycogenolysis and inhibit glucose utilization in peripheral tissues. Diabetic patients do not develop hyperglycemia in 1st hr after SI. At SI, BG values decrease, indicating inhibitory effect of spinal anaesthesia. The reason behind this finding can also be increased insulin levels due to exogenous supplementation which better attenuates the stress response. BG values increases after 2nd hr after SI owing to regression of effect of spinal anaesthesia. This finding is correlated by the finding of Moller et al14. This study also showed that the inhibitory effect of spinal anaesthesia on the stress response to surgery is transient, and correlates to the regression of sensory analgesia.
CONCLUSION Spinal anaesthesia blunts surgical stress response and hence, at SI, BG values decrease. But, BG values increase at other times in perioperative period owing to the regression of sensory analgesia.
REFERENCES
|
|
 Home
Home