Official Journals By StatPerson Publication
|
Table of Content Volume 6 Issue 2 - May 2018
A comparative study of Dexmedetomidine and Clonidine as an adjuvants to 0.5% Ropivacaine in upper limb surgeries done under Supraclavicular brachialplexus block
Sonali M Khobragade1*, Balkrishna Bagwale2, Sandhya Manjrekar3
1Associate Professor,2Resident, 3Professor and HOD, Department of Anaesthesiology, Indira Gandhi Government Medical College and Hospital, Nagpur, Maharashtra, INDIA. Email: drsonalibhagat@yahoo.in
Abstract Background: Regional anaesthetic techniques are highly effective in allevating pain during various surgical procedures. The role of peripheral nerve block has expanded from the operating room into the arena of postoperative and chronic pain management. Addition of adjuvants decreases the dose of local anaesthetics and thereby decrease the potential local anaesthetic toxicity. The objective of our study was to compare the clinical profile of Alpha two agonists Dexmedetomidine and Clonidine as an adjuvant to Ropivacaine in supraclavicular brachial plexus block with respect to sensory and motor blockade, level of sedation and duration of analgesia.. Material and Methods: Sixty adult patients of ASA Grade I and II posted for elective upper limb surgeries under Supraclavicular brachial plexus block were randomly divided into 2 groups. Group C: 29 ml of 0.5 % Ropivacaine with Clonidine 1 mcg/kg ( volume 30 ml). Group D: 29 ml of 0.5 % Ropivacaine with Dexmedetomidine 1 mcg/kg( volume 30 ml).assessment of sensory blockade was done by Hollmen scale whereas motor block assessment was done by Bromage Scale. Degree of pain was evaluated by Visual Analogue Scale. Level of sedation was assessed by 5 point sedation scale. Results: There was statistically significant difference between the groups for onset of sensory and motor blockade, time for complete sensory and motor block, total duration of sensory and motor blockade as well as for duration of analgesia. Sedation scale score and Visual Analogue Scale score except at 6 Hrs were comparable between the groups. Conclusion: Dexmedetomidine is a better adjuvant to Ropivacaine for supraclavicular brachial plexus block when compared to Clonidine as it provides earlier onset of sensory and motor blockade, prolongs the duration of blockade and postoperative analgesia withminimal side effects. Key Words: Dexmedetomidine, Clonidine, Ropivacaine, Supraclavicular brachial plexus block, Upper limb surgeries.
INTRODUCTION The International Association for the Study of Pain defines pain as an “unpleasant sensory and emotional experience associated with actual damage or potential tissue damage or described in terms of such damage.”1 From various decades, attempts were made to relieve the surgical pain by various means and techniques. Regional anaesthetic techniques are as successful as general anaesthesia in controlling pain during surgery. The role of peripheral nerve block has expanded from the operating suite into the arena of postoperative and chronic pain management. Peripheral nerve block are achieved by injecting local anaesthetic solution around a nerve root to produce anaesthesia in the distribution of that nerve without any distortion of the surgical anatomy.2 Halsted and Hall (1895) performed the first regional anaesthetic procedure and indeed performed the first operation under brachial plexus block when he freed the cords and nerves of the brachial plexus after blocking cervical roots in neck with cocaine solution.3,4 The first percutaneous supraclavicular block was performed in 1911 by german surgeon DiedrichKulenkampff(1880-1967)5.There are many advantages of a single shot peripheral nerve block like rapid onset, predictable and dense anaesthesia, a relatively simpler technique, good muscle relaxation and adequate post operative analgesia. It also means early ambulation, early oral intake, avoiding intubation and its complications with lesser systemic side effects and fewer postoperative side effects. Among the various peripheral nerve blocks, Brachial Plexus Block is one of the most commonly practiced blocks, as it offers almost complete anaesthesia and analgesia and an excellent operative field for surgeries of the upper extremities. The various local anaesthetics used in Supraclavicular block are quite effective but the duration of analgesia is a major limiting factor along with toxicity. Ropivacaine is a pure S[-] enantiomer, unlike bupivacaine which is a racemic mixture, developed for the purpose of reducing potential cardiovascular and central nervous system toxicity and improving relative sensory and motor block profiles.6 Addition of adjuvants decreases the dose of local anaesthetics and thereby decrease the potential local anaesthetictoxicity.There has always been a search for adjuvants which can be added to the local anaesthetics in peripheral nerve block to improve the duration and quality of analgesia but without producing any major adverse effects. Various studies have investigated several adjuncts, including opioids 7, clonidine8, neostigmine 9, hyaluronidase10 and dexamethasone11.Since their synthesis, α 2 adrenergic receptor agonists have been studied for their sedative, analgesic, perioperative sympatholytic and cardiovascular stabilizing effects with concomitant reduced anaesthetic requirements. They have been used intrathecally, epidurally or as part of peripheral nerve blocks in conjunction with local anaesthetics in an attempt to prolong the duration of analgesia and to improve the quality of the block.12,13Clonidine, is a selective α2 adrenergic agonist with some α1 agonist property. Clonidine possibly enhances or amplifies the sodium channel blockade action of local anesthetics by opening up the potassium channels resulting in membrane hyperpolarisation, a state in which the cell is unresponsive to excitatory input.14 Dexmedetomidine, the newer drug, is a potent α2adrenoceptor agonist, and about eight-times more selective towards the α2adrenoceptor than clonidine13. Probabaly peripherally α2 agonists produce analgesia by reducing the release of norepinephrine and also causing inhibitory effects on the nerve fibre action potentials which is receptor independent. Central analgesia and sedation by these drugs is caused by the inhibition of release of Substance P in the nociceptive pathway at the level of the dorsal root neurons and by activating the α2 receptors in the locus cerulus15. It has been used in various strengths as an adjunct to local anasthetics to prolong the duration of block and post-operative analgesia in various peripheral blocks.16,17 The anaesthetic and analgesic requirement of local anaesthetics gets reduced by the use of these two adjuvants because of their analgesic property and augmentation of local anaesthetics effects. Keeping all this into consideration, this current study was designed to study effects of Clonidine and Dexmedetomidineas an adjuvants to 0.5 % Ropivacaine in Supraclavicular brachial plexus block.
MATERIAL AND METHODS The present study was conducted in the Department of Anaesthesiology at a tertiary care centre during the period of October 2016 to October 2017. After approval from the Institutional ethics committee, this prospective, observational study was conducted to assess and compare the efficacy and clinical profile of alpha two agonists (Dexmedetomidine and Clonidine) used as an adjuvants to Ropivacaine in Supraclavicular brachial plexus block. The study was carried out in 60 adult patients admitted in the department of Orthopaedics, with age in the range of 18-60 years, ASA Grade I and II posted for elective upper limb surgeries under Supraclavicular brachial plexus block. They were included in the study only after obtaining a written informed consent. Inclusion Criteria
Patientswho had contraindications to peripheral nerve block like bleeding diathesis, local infection and patients on anticoagulants, patients with neurological lesions in the upper limb, diabetic neuropathy, psychiatric illness and neurological disease, cardiovascular diseases like arrhythmias, ischaemic heart disease and valvular heart disease, Liver, Respiratory, Kidney and Endocrine diseases and hemodynamically unstable patients were excluded from the study. Detailed pre-anaesthetic evaluation of the patients was performed by an anaesthesiologist a day before the surgery. Preliminary Investigations in the form of Complete blood count, Random blood sugar. Bleeding time, Clotting time, Coagulation profile, Liver function tests, Kidney function tests, Electrocardiography (ECG), Chest x ray postero-anterior (PA) view were noted.All patients were kept nil by mouth for 8 hrs. All patients were given overnight sedation in the form of Tab. Alprazolam 0.5 mg orally a day prior to surgery.In operation theatre, multipara monitoring device with ECG, pulse rate, non-invasive blood pressure, SpO2 was attached to the patient and baseline parameters were noted. Ringer lactate was started after establishing intravenous line with 18 G cannula in unaffected limb, before the block. Thereafter, intravenous fluids were calculated and given as per body weight and operative loss. Patients also received Inj. Ranitidine 50 mg and Inj. Ondansetron 4 mg IV slowly as a premedication.Patients were randomly divided into two groups of 30 each. Group C: 29 ml of 0.5 % Ropivacaine with Clonidine 1 mcg/kg (total volume 30 ml). Group D: 29 ml of 0.5 % Ropivacaine with Dexmedetomidine 1 mcg/kg (total volume 30 ml). Positioning of the patient: Patients were placed in supine position with head turned away from the side where block was performed. A pillow was placed below the shoulder to make landmarks prominent. The arm to be anaesthetized was adducted and hand should be extended along the side. By asking the patient to raise his head slightly, the lateral border of sternocleidomastoid was palpated. Palpating fingers then rolled over the belly of anterior scalene muscle into interscalene groove where mark was made 1.5 to 2.0 cm posterior to the midpoint of clavicle.Palpation of the subclavian artery at this site confirmed the landmark. TECHNIQUE: Under all aseptic precautions, the skin wheal was raised with 1ml of 2% Lignocaine. A nerve stimulator (Organon, Ireland) with 22G, 50 mm long stimulating needle (stimuplex, Germany) was used to locate the brachial plexus. The stimulating needle was connected with the Nerve stimulator, with the current output set at 1.0 mA and repeat twitch mode selected by the assistant under the guidance of an expert anaesthesiologist. A 22G stimuplex needle was inserted 1.5-2 cm posterior to the midpoint of clavicle. The needle was advanced caudal, slightly medial, and posterior direction until a motor response was elicited. A twitch of the upper trunk (shoulder) was considered as the evidence of the needle approaching the brachial plexus. Wrist flexion and extension of the fingers was taken as acceptable responses to nerve stimulator and the current was gradually reduced to 0.5 mA, whereby maintaining the visible twitches. The total volume of the anaesthetic solution was injected at an incremental dose of 5 ml each, preceded by negative aspiration of blood in each group. Time of injection was considered as Time -0.
Figure 1: Figure 2:
Intra operatively all patients were monitored for Heart rate, Blood pressure (systolic, diastolic and mean), Respiratory rate, SpO2, Sensory block : onset and duration by Hollmen Scale, Motor block: onset, duration and density of motor block using Bromage scale for upper extremity, Visual analogue scale score for pain assessment, Sedation score using Five point sedation scale.Intraoperatively and postoperatively, bradycardia (heart rate<60 beats per minute) was to be treated with 0.6 mg injection Atropine and hypotension (systolic blood pressure falling more than 20% basal value or less than 80 mmHg) with 3-6 mg injection Mephentermine as bolus along with necessary fluid replacement. Respiratory depression (SpO2 <90% or respiratory rate < 10 breaths per minute) if any, was to be treated by administering 100% O2 with face mask or ventilation with IPPV accordingly. Assessment of Sensory Block: Sensory block was evaluated by Hollmen scale22 and findings were recorded at an interval of every 2 min from time-0 till complete sensory block was achieved i.eHollmen Score = 4 Hollmen Scale22: Score 1 = Normal sensation of pinprick. Score 2 = Pin prick felt as sharp pointed but weaker compared with same area in the other upper limb. Score 3 = Pin prick recognized as touch with blunt object. Score 4 = No perception of pin prick. Onset Time of Sensory Block (OTSB): was taken as the time interval in minutes from time-0 till sensory block started appearing i.e. Hollmen score = 2. Time for Complete Sensory Block (TCSB): was taken as the duration of time in minutes from time-0 (Time of injection of local anaesthetic) till complete sensory block was achieved i.eHollmen Score=4. Thereafter effect of block was tested every 30 minutes. Total Duration of Sensory Block (TDSB): was taken as the duration of time in minutes from the time-0 till the time when patient came back to Hollmen score 1. Assessment of Motor Block:Motor block was evaluated by using Bromage Scale (BS)22 for upper extremity and findings were recorded at an interval of every 2 min from time-0 till complete loss of motor power was achieved i.e. BS Score=3 Bromage scale for upper extremity22: 0: Able to raise the extended arm to 90° for full 2 seconds. 1: Able to flex the elbow and move the fingers but unable to raise the extended arm. 2: Unable to flex the elbow but able to move the fingers. 3: Unable to move the arm, elbow and fingers Onset Time of Motor Block (OTMB): was taken as the time interval in minutes from time-0 (Time of local anaesthetic injection) till motor block started appearing i.e. BS score ≥1. Time for Complete Motor Block (TCMB): was taken as the duration of time in minutes from time-0 (Time of local anaesthetic injection) till complete motor block was achieved i.e. BS score=3. Thereafter effect of block was tested every 30 minutes. Total Duration of Motor Block (TDMB): was taken as the duration of time in minutes from time-0 ( Time of local anaesthetic injection) till the time when BS score 0 with complete recovery of motor functions in the postoperative period. ADEQUACY OF BLOCK:Adequacy of block was evaluated by Allis clamp test before handing over the patient to surgeon. The test was done by asking the patient whether they felt any discomfort when pressure applied with the Allis clamp at the area of the surgical field. The reading was recorded as follows:
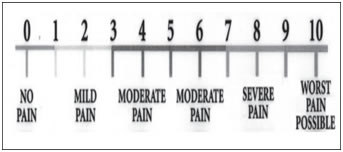
These patients were supplemented with intravenous fentanyl (1 mcg/kg) and midazolam (0.02mg/kg). Surgery was allowed to proceed when there was complete block or patient did not complained of pain at the surgical site by Allis clamp test after supplementation, in case of inadequate effect. Block was considered as a failure if complete sensory and motor block was not achieved even after 45 minutes. Failed blocks were converted to GA and these patients were excluded from the study. Haemodynamic changes: pulse rate, systolic blood pressure, diastolic blood pressure, mean arterial pressure were monitored. Level of Sedation: Level of sedation was assessed using Five Point Sedation scale22. Level of sedation was assessed at an interval of every 30 min from Time-0 till the end of surgery using the 5 point sedation scale22. The scoring was recorded as follows: 1= Awake and alert. 2= Sedated but responding to verbal stimulus. 3= Sedated, responding to mild physical stimulus 4= Sedated, responding to moderate or strong physical stimulus 5= Not arousable Duration of Complete Analgesia: Duration of post-operative analgesia was taken till the time patient asked for rescue analgesia i.e. VAS ≥4. Pain was assessed by Visual Analogue Scale (VAS). VAS was recorded and assesed at an interval of every 30 minutes till the score ≥ 4.Time of first dose of post–operative systemic analgesic was on the basis of VAS score ≥ 4 and was noted for use as duration of analgesia. Visual analogue scale18 (Figure 1):Visual analogue scale consists of a 10 cm line, marked at 1 cm each. The patiepatients a mark on the line that represents the intensity of pain he or she experienced. Mark “0” represents no pain and mark “10” represents worst possible pain. The numbers marked by the patient was taken as units of pain intensity. 0 = no pain 10= maximum pain
Figure 3:
Duration of surgery and type of surgical procedure done was recorded. Intra-operative and post operativecomplications were looked for like inadequacy of block, any reaction at injection site like haematoma, persistent bradycardia, persistent hypotension,oversedation- sedation score >4, any respiratory distress, pneumothorax, fall in respiratory rate to <10 per min, fall in SpO2 to < 90%, dryness of mouth, nausea, vomiting, local haematoma, any symptoms or signs of local anaesthetic toxicity, any significant ECG changes and Horner’s syndrome.Intra-operative medication given (if any) for sedation or management of complications was noted and recorded.Nausea and/or vomiting was treated with intravenous Ondensetron 4 mg slowly. After the completion of the surgery patients were shifted to post operative recovery ward without prescribing any analgesics in any form. Patients were monitored till the complete recession of sensory as well as motor block occured and till the time patient didnot demand any analgesic or VAS Score ≥ 4. For pain relief, patients were given systemic analgesic Inj. Diclofenec Sodium 1.5mg/kg (75mg) IV slowly or as per individual requirement. Subsequently analgesia was given with injection Diclofenac sodium 1.5mg/kg I.V. slowly BD along with injection Ranitidine hydrochloride 50mg twice daily. Parameters along with vitals were recorded in the post operative period every 6 hrly till 24 hrs after the block.Post operatively CXR was done after six hours from Time-0 or early if patient showed any clinical evidence of pneumothorax and finding was recorded and treated accordingly. Statistical Analysis:Data were collected, tabulated, coded then analysed using SPSS computer software version 20.0 and Microsoft word and Excel have been used to generate graphs and tables etc.
Table 1:
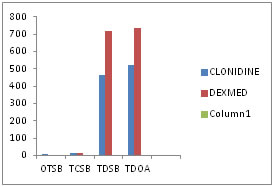
RESULTS Sixty patients included in the study were comparable in demographic characteristics such as age, weight and duration of surgery. SENSORY BLOCK CHARACTERISTICS(Figure 4): The mean onset time of sensory block was achieved significantly earlier in group D (4.97 ± 1.67 minutes) than in group C(8.60 ± 2.33 minutes)(p=0.000). The mean(SD) time for complete sensory block in Group D(12.0 ± 2.05 minutes) was earlier as compared to Group C(17.10 ±5.99 minutes) (p=0.000). The mean total duration sensory block in Group D (715.33 ± 53.79 minutes) was significantly prolonged as compared to Group C(462.67± 58.66 minutes) (p=0.000). The mean time for total duration of analgesia in Group D(734.00 ± 57.81 minutes) was prolonged as compared to Group C(519.00 ± 53.76 minutes) (p=0.000). Figure 4: Sensory block characteristics
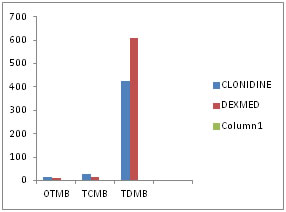
MOTOR BLOCK CHARACTERISTICS (figure 5): The mean (±SD) onset time of motor block in Group C(Clonidine) was 15.33 (± 5.38) minutes and in Group D (Dexmedetomidine) was 9.80 (± 4.25) minutes which was statistically significant(p=0.000). The mean time for complete motor block in Group D(16.93 ± 3.95 minutes) was earlier as compared to GroupC(26.43 ± 8.02 minutes) (p=0.000). The mean (± SD) total duration of motor block in Group C(Clonidine) was 426.17 (± 50.98) minutes and in Group D(Dexmedetomidine) was 608.43 (± 51.46) minutes which was statistically significant(p=0.000). Figure 5: Motor block characteristics
Table 2: Comparison of the mean (±sd) pain score (visual analogue scale) in the two groups (figure 6):
The pain scores were noted at periodic intervals till 24 Hrs postoperatively. The mean (SD) VAS Scores in Dexmedetomidine group were significantly lower than Clonidine group at 6 Hrs. while the mean VAS scores in Clonidine group and Dexmedetomidine group were comparable at various time intervals in rest of the perioperative period.
Table 3: Comparison of the mean (±sd) sedation score (figure 7).
The mean sedation scores were comparable between two groups at various time intervals.
Figure 6: Comparison of mean vas score Figure 7: Line diagram5 showing mean sedation score Table 4: Perioperative complications:
The hypotension and bradycardia was not observed in any patients of either group. No major alterations were observed in both the groups. Dry mouth was observed in 1 (3.3%) patient in Group D(Dexmedetomidine). Nausea was observed in 1(3.3%) patient in Group C(Clonidine).
DISCUSSION An increasing demand for regional anesthesia from patients and surgeons both matches the fact that regional anesthesia can provide superior pain management and improves patient outcome. Supraclavicular brachial plexus block is a very effective procedure of anaesthesia for various upper limb surgeries due to its effectiveness in terms of cost and performance, margin of safety and good postoperative analgesia.Over the period of years, there is advent of various sophisticated techniques in anaesthesia. Regional anaesthesia has various advantages over general anaesthesia like good postoperative pain relief, reduced cardiac complications in high risk patients, reduced bleeding and transfusion requirement and early postoperative ambulation.Supraclavicular approach of brachial plexus block gives an effective anaesthesia for all portions of upper extremity and is carried out at the level of trunks of brachial plexus. The plexus is blocked where it is most compact i.e. at the middle of brachial plexus, resulting in homogeneous spread of anaesthetic drug throughout the plexus with a fast onset and complete block.Supraclavicular block with local anaesthetic agents provide excellent operating conditions with good muscle relaxation, but the duration of analgesia is rarely maintained for more than 4-6 hours even with the longest acting local anaesthetic agents (Bupivacaine, Ropivacaine and Levo-bupivacaine). Continuous infusion of local anaesthetic agents into brachial plexus sheath requires an infusion pump and has potential for cumulative toxicity and unpredictable variability in absorption. Various studies have shown that addition of adjuvants like Clonidine and Dexmedetomidine in local anaesthetic solution in peripheral nerve blocks prolonged the duration of anaesthesia and analgesia.Alpha-2 adrenergic receptor agonists have been the focus of interest for their use as an adjuvants to local anaesthetic agents. Alpha-2 receptors are found in many sites throughout the body. Alpha-2 adrenoceptors are found in peripheral and central nervous systems, in effector organs such as the liver, kidney, pancreas, eye, vascular smooth muscles and platelets. Clonidine, is a selective α2 adrenergic agonist with some α-1 agonist property. Dexmedetomidine is a new alpha 2-agonist. It has sedative, analgesic, sympatholytic and anxiolytic effect that blunts many cardiovascular responses in the perioperative period. It causes conscious sedation without causing respiratory depression. Ropivacaine is a pure S (-) enantiomer, which has lower central nervous system and cardiovascular toxicity as compared to R (+) enantiomer(Bupivacaine). The incidence of cardiotoxicity and CNS toxicity of Ropivacaine is lower than that of Bupivacaine due to its stereoselective properties and less lipophilicity.In the present study, the two groups were comparable with respect to age, weight. ASA grading and duration of surgery. The findings from several studies are consistent with various findings from our study. Total Duration Of Sensory Block (TDSB): In our study, the mean (±SD) total duration of sensory block in Group C (Clonidine) was 462.67(±58.66) minutes and in Group D (Dexmedetomidine) was 715.33(±53.79) minutes. The mean (±SD) total duration of sensory block was significantly prolonged in Group D(Dexmedetomidine) than Group C(Clonidine) (p=0.000) (HS). AgarwalSandhya et al (2014)25carried out a study to compare the effects of Dexmedetomidine(100µg) as an adjuvant to 0.325% Bupivacaine in supraclavicular brachial plexus block using the peripheral nerve stimulator. They found that, the mean (±SD) total duration of sensory block in Control group was 234.8 (±47.9) minutes and in Dexmedetomidine group was 755.6 (±126.8) minutes. The mean total duration of sensory blockwas significantly prolonged in Dexmedetomidine group than Control group (p < 0.001). More Preeti et al (2015)21found that, the mean (±SD) total duration of sensory block in Clonidine group was 470.33(±55.67) minutes and in Dexmedetomidine group was 690.00(±87.41) minutes. The mean total duration of sensory block was significantly prolonged in Dexmedetomidine group than Clonidine group (p < 0.05). Sebastian Don et al (2015)24found that, the mean (±SD) total duration of sensory block in Clonidine group was 463.5(±40.32) minutes and in Dexmedetomidine group was 647.67(±49.85) minutes. The mean total duration of sensory blockwas significantly prolonged in Dexmedetomidine group than Clonidine group (p < 0.01). Onset Time Of Motor Block (OTMB):In our study, the mean (±SD) onset time of motor block in Group C (Clonidine) was 15.33 (±5.38) minutes and in Group D (Dexmedetomidine) was 9.80(±4.25) minutes. Onset time of motor block was significantly earlier in Group D (Dexmedetomidine) than Group C(Clonidine) (p=0.000) (HS). Kirubahar R et al (2016)19found that the mean (±SD) onset of motor block in Clonidine group was 13.1(±1.42) minutes and in Dexmedetomidine group was 9.63(±0.89) minutes.The mean onset time of motor block was earlier in Dexmedetomidine group than Clonidine group which was statistically significant (p <0.001). More Preeti et al (2015)21found that the mean (±SD) onset of motor block in Clonidine group was 15.17(±1.76) minutes and in Dexmedetomidine group was 12.63(±2.19) minutes.The mean onset time of motor block was earlier inDexmedetomidine group than Clonidinegroup. This difference was statistically significant (p<0.05).BafnaUsha et al (2015)20found that the mean (±SD) onset of motor block in Clonidine group was 12.1(±4.1) minutes and in Dexmedetomidine group was 8.9(±1.41) minutes.The mean onset time of motor block was earlier inDexmedetomidine group than Clonidinegroup. This difference was statistically significant (p < 0.0001). Time for Complete Motor Block (TCMB): In our study, the mean (±SD) time for complete motor blockin Group C (Clonidine) was 26.43(±8.02) minutes and in Group D (Dexmedetomidine) was 16.93(±3.95) minutes. The mean (SD) time for complete motor block was significantly earlier in Group D (Dexmedetomidine) than Group C (Clonidine) (p=0.000) (HS). AgarwalSandhya et al (2014)25found that, the mean (±SD) time for complete motor block in Control group was 22.7(±2.8) minutes and in Dexmedetomidine group was 16.3(±1.7) minutes. The mean time for complete motor block was significantly earlier in Dexmedetomidine group than Control group (p<0.001).JinjilKavitha et al (2015)22found that, the mean (±SD) time for complete motor block in Clonidine group was 24.4(±1.5) minutes and in Dexmedetomidine group was 19.9(±1.7) minutes. The mean time for complete motor block was significantly earlier in Dexmedetomidine group than Clonidine group (p<0.05). Total Duration of Motor Block (TDMB):In our study, the mean (±SD) total duration of motor block in Group C (Clonidine) was 426.17(±50.98) minutes and in Group D (Dexmedetomidine) was 608.43(±51.46) minutes. The mean (±SD) total duration of motor block was significantly prolonged in Group D (Dexmedetomidine) than Group C(Clonidine) (p=0.000) (HS).Sebastian Don et al (2015)24 found that, the mean (±SD) total duration of motor block in Clonidine group was 424.33 (±44.65) minutes and in Dexmedetomidine group was 600.83 (±46.722) minutes. The mean total duration of motor blockwas significantly prolonged in Dexmedetomidine group than Clonidine group (p <0.01).Patki Y S et al (2015)26found that, the mean (±SD) total duration of motor block in Control group was 462.83 (±15.01) minutes and in Dexmedetomidine group was 608.33 (±10.23) minutes. The mean total duration of motor block was significantly prolonged in Dexmedetomidine group than Control group (p <0.001). Duration of Analgesia: In our study, the mean (±SD) duration of analgesia in Group C (Clonidine) was 519.00(±53.76) minutes and in Group D (Dexmedetomidine) was 734.00(±57.81) minutes. The mean (SD) duration of analgesia was significantly prolonged in Group D (Dexmedetomidine) than Group C (Clonidine) (p=0.000) (HS).More Preeti et al (2015) 21found that, the mean (±SD) duration of analgesia in Clonidine group was 516.00 (±45.15) minutes and in Dexmedetomidine group was 721. 33(±88.27) minutes. The mean duration of analgesia was significantly prolonged in Dexmedetomidine group than Clonidine group (p <0.05).Sebastian Don et al (2015)24found that, the mean (±SD) duration of analgesia in Clonidine group was 510.83(±42.30) minutes and in Dexmedetomidine group was 720.83 (±44.16) minutes. The mean duration of analgesia was significantly prolonged in Dexmedetomidine group than Clonidine group (p <0.01). Complications:In our study, dry mouth was observed in 1(3.3%) patient of Group D (Dexmedetomidine).Nausea was observed in 1(3.3%) patient of Group C (Clonidine). None of the patient in either group had bradycardia, hypotension, Horner’s syndrome, pneumothorax, respiratory depression, any neurological complications, oversedation, local hematoma and local anaesthetic toxicity. More Preeti et al (2015) 21found that, patients in both the groups suffered nausea and vomiting (5 patients in Dexmedetomidine group and 2 patients in Clonidine group ). Dryness of mouth was observed in 1 patient in Dexmedetomidine group and blurring of vision was observed in 1 patient in Clonidine group. JinjilKavitha et al (2015) 22observed no complications in Clonidine group and Dexmedetomidine group. None of the patients developed any serious complications due to block procedure (pneumothorax, large haematoma, Horners syndrome, prolonged nerve palsy, nausea, vomiting or dry mouth).BafnaUsha etal (2015)20observed that, there was hypotension in 1(2.5%) patient and bradycardia in 2 (5%) patients in Dexmedetomidine group.In our study, incidence of adverse effects were minimal and managed accordingly.
CONCLUSION From the present study, we conclude that Dexmedetomidine is a better adjuvant to Ropivacaine for supraclavicular brachial plexus block when compared to Clonidine as it provides earlier onset of sensory and motor blockade, prolongs the duration of sensorimotor blockade and postoperative analgesia with stable vitals and minimal side effects.
REFERENCES
|
|
 Home
Home