Official Journals By StatPerson Publication
|
Table of Content Volume 4 Issue 1 - October 2017
Evaluation of cilnidipine as bronchial smooth muscle relaxant in guinea pigs
M D Sibgatullah1, Satish A M2, Shruthi S L3*
1Drug safety Physician, Vigi Medsafe Pvt Ltd, Hyderabad, Telangana, INDIA. 2Associate Professor, Department of Pharmacology, JSS Medical College, Mysusru, Karnataka, INDIA. 3Tutor, Department of Pharmacology, Shimoga Institute of Medical Sciences, Shimoga, Karnataka, INDIA. Email: drshruthideepak@yahoo.com
Abstract Background: Chloride (Cl-), being the most abundant permeable anion, plays an important role in various cellular functions. Impairment in the transport of Cl- is known to cause epilepsy, cystic fibrosis, myotonia, lysosomal storage disease, deafness, renal stones, and osteoporosis Methodology: Healthy adult guinea pigs of either sex from animal house Medical College were used for study, Pregnant and diseased guinea pigs were excluded. The body weight of these animals varied from 400 to 600 grams. Results: The mean PCD time for the control group is 60.5 sec with a standard deviation of ± 2.42 seconds. The mean PCD time for the standard group treated by oral salbutamol in the dose of 4.5mg/kg body wt. is 371 seconds with a standard deviation of ±10.41 seconds. Conclusion: The tests drugs, i.e. Cilnidipine and Amlodipine showed a significant increase in the PCD time after exposure to Acetyl choline when compared to the control group. Key Words: Cilnidipine, Bronchial Smooth Muscle Relaxant, Guinea pigs.
Asthma is a chronic inflammatory disorder of the airways characterized by acute exacerbation of coughing, dyspnoea, and wheezing and chest tightness particularly at night as well as at the early morning1. Asthma was recognized in ancient Egypt and was treated by drinking an incense mixture known as kyphi2. It was officially named as a specific respiratory problem by Hippocrates circa 450 BC, with the Greek word for “panting “forming the basis of our modern name3. In 200 BC it was believed to be at least partly related to the emotions. Asthma is also widely recognized as a disease of lung characterized by reversible bronchoconstriction, elevated basal airway tone and lymphocyte (eosinophilis) activation and accumulation, epithelial cell dysfunction and damage, smooth muscle and sub mucosal gland hypertrophy, sub mucosal fibrosis, airway wall edema, mucous overproduction and episodes of nonspecific airway hyper- responsiveness to spasmogens4. Asthma is caused by a combination of complex and incompletely understood environmental and genetic interactions. These factors influence both its severity and its responsiveness to treatment. It is believed that the recent increased rates of asthma are due to changing epigenetic (heritable factors other than those related to the DNA sequence) and a changing living environment5. While asthma is a well-recognized condition, there is not one universal agreed upon definition3. It is defined by the Global Initiative for Asthma as "a chronic inflammatory disorder of the airways in which many cells and cellular elements play a role. The chronic inflammation is associated with airway hyper-responsiveness that leads to recurrent episodes of wheezing, breathlessness, chest tightness and coughing particularly at night or in the early morning. These episodes are usually associated with widespread but variable airflow obstruction within the lung that is often reversible either spontaneously or with treatment"5. There is currently no precise test with the diagnosis typically based on the pattern of symptoms and response to therapy over time3. A diagnosis of asthma should be suspected if there is a history of: recurrent wheezing, coughing or difficulty breathing and these symptoms occur or worsen due to exercise, viral infections, allergens or air pollution. Spirometry is then used to confirm the diagnosis. In children under the age of six the diagnosis is more difficult as they are too young for spirometry6. Chloride (Cl-), being the most abundant permeable anion, plays an important role in various cellular functions. Impairment in the transport of Cl- is known to cause epilepsy, cystic fibrosis, myotonia, lysosomal storage disease, deafness, renal stones, and osteoporosis7.Chloride channels and transporters tightly regulate and mediate the movement of chloride through the cell membrane. Chloride channels can be grouped into four categories: ligand-gated chloride channels, voltage-activated chloride channels, cAMP-regulated chloride channels, and calcium-activated chloride channels (CaCCs)8. CaCCs are located on lung and gastrointestinal epithelial cells, smooth muscle cells, pace-making cells in GI tracts, and sensory neurons. They play an important role in electrolyte and fluid secretion for mucous hydration and protection against infection. Their location on apical membrane of airway epithelial cells, airway smooth muscle cells - well documented. Animal Selection: Healthy adult guinea pigs of either sex from animal house Medical College were Used for study, Pregnant and diseased guinea pigs were excluded. The body weight of these animals varied from 400 to 600 grams. Preparation of Animals: Guinea pigs (400-600 g) of either sex housed in standard Conditions of temperature (22 ±} 2°C), relative humidity (55 ±} 5%) and light (12 hours light/dark cycles) were used. They were fed with standard pellet diandnd provided water ad libitum. The experimental protocol was approved by Institutional Animal Ethical Committee as per the guidance of CPCSEA. A minimum of six animals were used in each group. Throughout the experiments, animals were processed according to the suggested ethical guideline for the care of laboratory animals. The drug was weighed and dissolved in sufficient amount of water to prepare required concentration Chemical and drugs: Group1 Control: Normal saline P.O - 10ml/kg Group2 standard: Salbutamol P.O - 4.5mg/kg Group3 test drug 1: Amlodipine P.O – 1.5 mg/kg Group4 test drug 2: Cilnidipine P.O – 1.5 mg/kg Models Inducing asthma by Histamine model: Experimental bronchospasm was induced by exposing the animals to histamine acid phosphate 0.25% under a constant pressure (40 mm/Hg) from the inbuilt nebulizer of the histamine chamber. The animals exposed to histamine aerosol showed progressive dyspnea. The end point pre convulsive dyspnea (PCD) was determined from the time of aerosol exposure to the onset of dyspnea leading to appearance of convulsion. Inducing asthma by Acetylcholine model: Experimental bronchial asthma was induced in guinea pigs by exposing them to 10% acetylcholine chloride under constant pressure (40mm/Hg) in an aerosol chamber85. The animals exposed to acetylcholine aerosol showed progressive dyspnoea. The end point pre convulsive dyspnoea (PCD) was determined from the time of aerosol exposure to the onset of dyspnoea leading to the appearance of convulsions.
RESULTS The evaluation study of anti-asthmatic effect of Calcium channel blockers Amlodipine and Cilnidipine in guinea pigs was done by using aerosol chamber. 24 guinea pigs of weight 450gms were selected and were divided into four groups each containing 6 guinea pigs (i.e., group I control, II standard, III Cilnidipine and IV amlodipine respectively). The pre-convulsive time of each guinea pig is recorded by exposing to 0.25% histamine aerosol and 10% acetyle choline chloride. Table 1: Individual PCD time of various groups under acetylcholine induced bronchospasm model, their means and standard deviations. In seconds
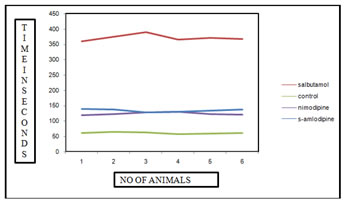
Figure 1: Graphical representation of PCD time for various test drugs in comparison with standard group and control group in acetyl choline induced bronchospasm method. In seconds Table 2: Individual PCD time of various groups under histamine induced bronchospasm model, their means and standard deviations. In seconds
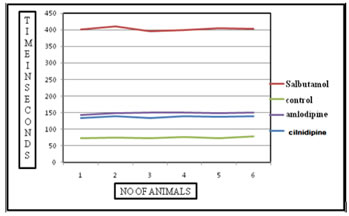
Figure 2: Graphical representation of PCD time for various test drugs in comparison with standard group and control group in histamine induced bronchospasm method. DISCUSSION This indicates the individual PCD time of various groups under acetylcholine induced bronchospasm model, their means and standard deviations. The mean PCD time for the control group is 60.5 sec with a standard deviation of ± 2.42 seconds. The mean PCD time for the standard group treated by oral salbutamol in the dose of 4.5mg/kg body wt. is 371 seconds with a standard deviation of± 10.41 seconds. The tests drugs, i.e. Cilnidipine and Amlodipine showed a significant increase in the PCD time after exposure to Acetyl choline when compared to the control group. The mean PCD time for Cilnidipine was 123.5seconds with a standard deviation of ± 3.89seconds and the PCD time for Amlodipine was 133.83 seconds with a standard deviation of ±4.56 seconds. The PCD time between the individual drugs was not significant when compared to each other indicating not much of difference of activity among these two calcium channel blockers. This indicates the individual PCD time of various groups under histamine induced bronchospasm model, their means and standard deviations. The mean PCD time for the control group is 74 sec with a standard deviation of ± 2.28 seconds. The mean PCD time for the standard group treated by oral salbutamol in the dose of 4.5mg/kg body wt. is 402 seconds with a standard deviation of± 5.21 seconds. The tests drugs, i.e. Cilnidipine and Amlodipine showed a significant increase in the PCD time after exposure to histamine when compared to the control group. The mean PCD time for Cilnidipine was 137.5seconds with a standard deviation of ± 2.42seconds and the PCD time for Amlodipine was 148.16 seconds with a standard deviation of ±3.25 seconds. The PCD time between the individual drugs was not significant when compared to each other indicating not much of difference of activity among these two calcium channel blockers. Smooth muscles depolarize by inward Ca2+ movement through voltage sensitive channels. Ca2+ influx triggers release of more Ca2+ from intracellular stores leading to excitation-contraction coupling through phosphorylation of myosin light chain. Spontaneous depolarization by Ca2+ sparks turn on Ca2+ activated Cl- channels causing spontaneous transient inward currents (STICs) resulting in membrane depolarization and sustain smooth muscle contraction. There is also a possibility that binding of an auxiliary protein to CaCC, mediates its activation upon an increase in intracellular calcium levels. Calmodulin, a small protein which has submicromolar affinity to calcium, undergoes dramatic conformational change upon binding calcium and has been postulated to carry out such a function. Calmodulin being well known to be the calcium sensor for the small-conductance calcium-activated potassium channels9, it is tempting to envision a similar scenario for the calcium-activated chloride channel. Amlodipine by reducing availability of intracellular Ca2+, prevents spontaneous depolarizations by Ca2+ sparks, thereby preventing activation of CaCC. However further studies are warranted to develop compounds that potently and specifically modulate CaCCs.
CONCLUSION Amlodipine and Cilnidipinehas got a significant bronchial smooth muscle relaxant activity when compared to the control group. Amlodipine and Cilnidipinehas got a similar profile of action when compared to each other and there is no significant difference in their activity when compared to each other.
REFERENCES
|
|
 Home
Home