Official Journals By StatPerson Publication
|
Table of Content Volume 6 Issue 1 - April 2018
Efficacy and safety of oral fluconazole vs topical antifungal imidazoles in tinea corporis: A comparative therapeutic trial
Mohamed Salim Valiyakathoy1, Soopy Kayanaduth2*, Roopashree Gopinath3
1Assistant Professor, Department of Pharmacology, Government Medical College, Kozhikode, Kerala, INDIA. 2Associate Professor,Department of General Medicine, Government Medical College, Kozhikode, Kerala, INDIA. 3Kaplan University, Davenport, Iowa, USA. Email:drsoopy@gmail.com
Abstract Background:Dermatophytes are the most common causes of superficial fungal infections worldwide. All dermatophytosis of glabrous skin except the palms, soles and groin are categorized under tinea corporis. Dermatophytic infections may be treated with both topical and systemic therapies. The present study aims to assess the efficacy and safety of oral fluconazole in comparison to topical antifungal miconazole nitrate and ketoconazole against tinea corporis.Methodology:This study is an open labelled comparative trial. About 60 patients (n =60) with dermatophytosis attending outpatient clinic of dermatology department of Government Medical College, Kozhikode, Kerala, India, were selected randomly. The diagnosis and prognosis was done by clinical and microscopic examination by mounting skin scrapings in 10% KOH solution. The comparison of efficacy was done by chi-square test. The cure rate in each group was expressed as percentage.P value of <0.05 was considered significant and <0.01 as highly significant.Results: The cure rate in tinea corporis with oral fluconazole therapy at the dose of 150 mg weekly for 3 weeks was 100 %. The cure rate observed with miconazole and ketoconazole were 60% and 53% respectively.Fluconazole treatment does not resulted in serious adverse effects and safer for short term therapy of tinea corporis.Conclusion: Oral fluconazole is more effective than topical antifungal agents like miconazole nitrate and ketoconazole for the treatment of tinea corporis. Key Word:dermatophytosis, tinea corporis, fluconazole,miconazole,ketoconazole.
INTRODUCTION Dermatophytosis refers to superficial fungal infection of the skin, hair and nail caused by keratinophilic fungi calleddermatophytes.Dermatophytes are the most common causes of superficial fungal infections worldwide. They are classified into threegroups based on their genera: a). Trichophyton - causes infections on skin, hair, and nails. b). Epidermophyton -causes infections on skin and nails. c). Microsporum- causes infections on skin and hair. Depending on whether a species be located predominantly in the soil, on animals or on humans, it is said to be geophilic, zoophilic or anthropophilic respectively. Furthermore, based upon the affected site, these have been classified clinically into tinea faciei (face),tinea capitis (head), tinea barbae (beard), tinea manus (hand), tinea corporis (body), tinea cruris (groin), tineapedis (foot), and tinea unguium (nail)1,2. Typical dermatophytosis have an annular appearance that is commonly referred to as ringworm or tinea1. Tinea corporis denotes to all dermatophytosis of glabrous skin except the palms, soles and groin3.All species of dermatophytes belonging to the genera Trichophyton, Microsporum or Epidermophyton can cause tinea corporis. The four most common dermatophytes that cause tinea corporis are Trichophyton rubrum, Trichophyton mentagrophytes, Microsporumcanis and Trichophyton tonsurans.In India, T. rubrum accounts for the majority of cases of tinea corporis4,7. The high environmental temperature and relative humidity of tropical and subtropical countries like India, particularly favors in precipitating tinea corporis. The higher incidence of tinea corporis is also associated with increased urbanization including the use of occlusive footwear and clothing. Tinea corporis may be transmitted by direct contact with other infected individuals or by infected animals8. Dermatophytic infections may be treated with both topical and systemic therapies. Topical therapy is generally effective for uncomplicated tinea corporis of small areas9. Several topical antifungals of different groups such as azole derivatives, allylamines,benzylamines, morpholine, etc. are effective in localized dermatophytoses. Most commonly used topical antifungal therapy are based on the use of imidazoles, such as clotrimazole, miconazole, and ketoconazole10. Whereas, systemic drugs, liketerbinafne and itraconazole, are widely used for the treatment of severe and chronic dermatophytosis11.Azole group of antifungal drugs include both imidazoles and triazoles. Miconazole and ketoconazole,belongs to the chemical class of imidazoles, are known for its proven antifungal activity. Miconazole has been used to treat superficial fungal infections since the early 1970s12,14. Ketoconazole is also an anti-fungal agent that has been topically used to stop the growth of dermatophytes15.Fluconazole, is a bis-triazole broad spectrum antifungal agent available as oral tablet, oral suspension and intravenous formulation. Like other imidazoles, fluconazole inhibits sterol incorporation into fungal cell walls and exerts the antifungal effects16. Although, fluconazole, miconazole and ketoconazole are used for the treatment of tinea corporis, there is paucity in information of comparative study regarding the clinical efficacy of oral fluconazole and topical imidazoles. Thus the present study aims to assess the efficacy and safety of oral fluconazole in comparison to topical antifungal miconazole nitrate and ketoconazole against tinea corporis. MATERIALS AND METHODS This study was an open labelled comparative trial conducted in accordance with the declaration of Helsinki and was approved by the Ethics committee of Government Medical College, Kozhikode. It was conducted in the outpatient clinic of the Dermatology Department, Government Medical College, Kozhikode, Kerala, India. A total of 60 (n=60) patients were included in this study. Written informed consent was taken from all the subjects. The diagnosis and treatment of Tinea corporis was done with the help of consultant dermatologist. Patient selection Inclusion criteria Tinea corporis is one of the most prevalent dermatophytosis in Kozhikode and the patients suffering from chronic, recurrent and wide spread lesions of tinea corporiswere included in the study. Exclusion criteria Diabetic and other immunocompromised patients, all mixed infections with candidiasis and patients taking medications for concomitant diseases were excluded. Study procedure About 60 patients (both male and female) with dermatophytosis attending outpatient clinic of dermatology department were selected randomly. The description of baseline parameters of patients included in the study is given in table 1. The diagnosis was done by clinical and microscopic examination by mounting skin scrapings in 10% KOH solution.
Table 1: Description of baseline parameters of patients included in the study
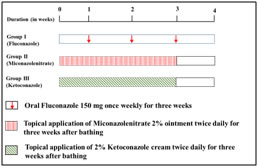
Figure 1: Experimental design The patients were grouped into three: - Group I consisted of 30 patients and they were treated with oral fluconazole 150mg once weekly for three consecutive weeks. Group II consisted of 15 patient and they were treated after bathing with topical application of Miconazole nitrate 2% ointment twice daily for three consecutive weeks. In group III15patientswere included. They were treated after bathing with topical application of 2% ketoconazole cream twice daily for three consecutive weeks. There was a regular weekly follow up for four weeks and also after two months. The clinical end points recorded were reduced itching, reduced erythema and disappearance of inflammatory signs and reappearance of normal skin colour. Clinical cure was also confirmed by microscopic examination of skin scrapings mounted in 10% KOH solution. The adverse effects reported were also recorded. All the medicines for this study were provided to the patient free of cost from the hospital pharmacy. Patient compliance was assessed from history and pill count only. Definition of clinical end points
Statistical Analysis The comparison of efficacy of short term oral fluconazole with topical antifungal imidazoles was done by chi-square test. The cure rate in each group was expressed as percentage.P value of <0.05 was considered significant and <0.01 as highly significant.
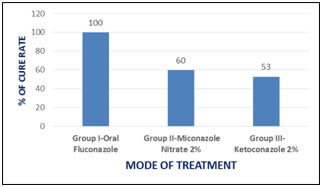
RESULTS All the 30 patients of Tinea Corporis in Group I were cured completely within 3 weeks of oral fluconazole treatment and no relapse reported after two months. Serious adverse effects were not reported. However, intense itching was noted in 20 patients after one week of therapy with oral fluconazole, but it was reduced in the second week. Mild abdominal discomfort and head ache were also reported(table 2). This shows that oral fluconazole is very effective and safer in Tinea corporis. The overall cure rate in Tinea Corporis with oral fluconazole therapy at the dose of 150mg weekly for 3 weeks was 100%. The P value is <0.01. 15 patients were studied in group II. Topical miconazole nitrate - 2% cream was applied over the lesions twice daily for three weeks. Out of the 15 patients with Tinea corporis, 9 patients were cured completely within two weeks and no relapse was reported further from these patients. Whereas, relapse was seen in 3 other patients after complete cure. Remaining 3 patients did not show any response to the treatment. The overall cure rate in Tinea Corporis with topical miconazole nitrate therapy was 60%. In group III, fifteen patients were treated with topical application of ketoconazole 2%, twice daily for three weeks. Out of the 15 patients with tinea corporis treated with topical application of ketoconazole, 8 patients were cured within 1 week and no relapse occurred. But 7 patients did not shown any improvement. The overall cure rate in tinea corporis with topical application of ketoconazole therapy was 53%. Figure 2 depicts the assessment of cure rate of tinea corporis to oral fluconazole, topical miconazole nitrate and topical ketoconazole. Table.2: Response of tinea corporis to the drugs used
Figure 2: Assessment of cure rate DISCUSSION Dermatophytosis, particularly tinea corporis, has been successfully treated with a variety of topical drug formulations once or twice daily for 1 to 6 weeks. The topical antifungals are usually most effective for localized infections. Oral agents approved for tinea corporisinclude gresofulvin, ketoconazole, itraconazole and fluconazole. They are used in widespread, chronic or recurrent conditions17. All oral agents except fluconazole produce several adverse effects and hepatotoxicity is prominent amongst them18. Fluconazole is used orally for treatment of tinea versicolor, vaginal and oral candidiasis, short term treatment of dermatophytosis and long term treatment of distal subungual onychomycosi19.Del Aguilaet al., reported that oral doses of 150mg fluconazoleonce weeklyfor 3-4 weeks in multicenter open label study on 70 patients in the treatment of tinea pedis produced 77% cure rate20.Stary and Sarnowinvestigated the efficacy offluconazole in the treatment of tinea corporis and tinea cruris in a single center open label study. 100 patients studied with same dosing schedule of fluconazole showed 71% cure rate21.In a double-blind, parallel group study Faergemannet al.,compared fluconazole 150mg once weekly with griseofulvin 500mg once daily for 4-6 weeks in the treatment of tinea corporis or tinea cruris. According to their findings, fluconazole 150mg once weekly for 6 weeks was both clinically and mycologically effective in the treatment of tinea corporis and tinea cruris. The adverse events reported were also less for fluconazole22.In the present study, short term oral fluconazole was compared with 2% ketoconazole cream and Miconazole nitrate 2% ointment in an open label fashion. Fluconazole was found to be effective in the treatment of tinea corporis when compared to ketoconazole and miconazole. The cure rate in the treatment of tinea corporis with oral fluconazole was 100%and no relapse was reported with the treatment.The antifungal activity of fluconazole is accomplished by preventing fungal membrane sterolsynthesis through the inhibition of cytochrome P450 (CYP) dependent lanosterol C-14α- demethylase conversion of lanosterol to ergosterol, resulting in an impairment of fungal cell replication. Even though CYP is present in mammalian cells, fluconazole is highly selective for fungal CYP. Fluconazole is well absorbed orally with extensive bioavailability, and most of the drug is excreted unchanged in the urine; only 11% is excreted as metabolites, while a small percentage is excreted in the feces. The elimination half-life of the drug is about 30 h (range 20–50 h)16.Once weekly fluconazole at 150mg has the potential advantage of causing less adverse events and interactions. Nausea, head ache, skin rash, vomiting, abdominal pain and diarrhoea are the most common adverse reactions occurring in 1-4% of patients. Hepatocellular dysfunction manifested by elevated LFT is the most common laboratory abnormality23. In this study the adverse effects observed with fluconazole were only head ache, itching and abdominal discomfort. The reason for mild adverse effects observed in this study may be due to the short term therapy (3 weeks) of oral fluconazole. In our study, the cure rate observed with miconazole and ketoconazole were 60% and 53% respectively. Relapse was seen when the topical antifungals were used for the treatment of tinea corporis. Miconazole is known to interfere with the synthesis of fungal and bacterial lipid membranes as it restrains the synthesis of ergosterol which results in accumulation of toxic methylated sterol intermediates in membranes and subsequently in fungal cell growth arrests. Another mode of miconazole antifungal action is the generation of reactive oxygen species in susceptible fungi and thereby inducing oxidative damage that kills the fungal cell. Side effects are rare if miconazole is applied topically12. Ketoconazole also inhibits the fungal cytochrome P450 enzyme systems that is necessary for synthesizing steroids (ergosterol), an essential component of the fungal cell membrane24. The topical antifungals often failed to respond in widespread and chronic lesions. In such situations oral antifungals are being used and found to be effective25. This may be the reason for lower efficacy of topical antifungals especially with ketoconazole in the present study, which showed only 53% cure rate in tinea corporis.Furthermore, intense itching was observed with the application of topical antifungals.
CONCLUSION Tinea corporis is found to be prevalent in Kozhikode, Kerala and that too in males due to high perspiration, lack of personal hygiene and using high alkaline soaps. Based on the studies reviewed and from the present study, it does appear that oral fluconazole is more effective than other topical antifungal agents for the treatment of tinea corporis.
REFERENCES
|
|
 Home
Home