|
Table of Content - Volume 14 Issue 1 - April 2020
Sub-maximal exercise as an indirect bronco-provocation tool to detect early airway dysfunction in asymptomatic smokers
Ketaki Poorey1, Manish Lamoria2*, Elvy Oommen3, Sushil Sharma4
1Assistant Professor,2Associate Professor, 4Tutor, Department of Physiology, Jaipur National University Institute for Medical Sciences and Research Centre, Jaipur, Rajasthan, INDIA. 3Professor & HOD, Department of Physiology, Parul Institute of Medical Sciences and Research, Vadodara, Gujarat., INDIA. Email: mhlamoria@gmail.com
Abstract Background: Smoking is the leading cause of chronic obstructive pulmonary disease (COPD). Most of the smokers remain asymptomatic and even have a normal spirometry for years but the truth is that the disease has already started. Indirect bronchoprovocation test like exercise stress test may become a useful tool to uncover this impending airway dysfunction. Aim of our study was to compare airway hyper-responsiveness and all the parameters of Forced Expiratory spirogram (FES) before and after exercise in healthy young volunteers and asymptomatic smokers respectively. Method. Spirometry was done at rest, and then volunteers were asked to exercise on the treadmill, so that, they achieved 80-90% of the maximum predicted heart rate for 5 minutes. Parameters of FES were recorded immediately after completion of the exercise challenge and subsequently at 5, 10 and 20 minutes of recovery period. Results It was found that bronchoprovocation test resulted in increase of FEV1 for the healthy volunteers while decrease in FEV1 in asymptomatic smokers after exercise challenge (3.49±0.7.42 Vs -12.17±12.03, p<0.0001). Similar trends were seen in all the flowrates. Key Words: bronchoprovocation, FEV, COPD, FES, smokers, spirometry, exercise challenge test.
INTRODUCTION Exercise is a common physiological stress 1. During exercise, respiratory system adapts to the increasing demand of O2 by increasing the rate and depth of respiration. It is a powerful physiologic stimulus for bronco-dilation. The effects of exercise have been addressed by many studies in healthy volunteers but use of exercise as a bronco-provocation tool in asymptomatic smokers has rarely been talked about. Exercise mediated bronchodilation in normal individuals is most likely to occur in peripheral airways because they are more compliant. Thus, peripheral airways are one of the determinant sites of ventilatory function in the human lung during physical activity 2. These are also the initial sites of abnormality in case of smokers developing Chronic Obstructive Pulmonary Disease (COPD). There have been a lot of articles published regarding the changes in the different parameters of spirometry in chronic smokers but less literature is available for the once who have been smoking since less than 5 years and have apparently no health issues. Chronic obstructive pulmonary disease (COPD) is a leading cause of morbidity and mortality in the world and its prevalence is rising day by day. Approximately 30% of smokers do not show chronic symptoms or abnormal lung function. Though, these so-called "healthy smokers" show some changes in lung morphology, and signs of lung inflammation. Smoking, apparently, always affects the lungs, although the extent and severity of these changes differ 3. In our study we wanted to know that, do such “healthy smokers” or better to say asymptomatic smokers with normal spirometry at rest, have a similar response to exercise challenge as the healthy volunteers do. Since post exercise bronchodilation is most in the peripheral airways which is also the site for obstruction in COPD, exercise challenge can become an integral part in identifying early airway disease in smokers who are otherwise healthy (4). It can warn the smoker in time to take measures when the changes may be still reversible.
MATERIAL AND METHOD The study was carried out in Department of Physiology, Baroda Medical College, Vadodara. Total 48 male subjects, with age between 18 to 25 years participated in the study. Out of which 24 were asymptomatic tobacco smokers and 24 were non-smokers. Subjects consisted of volunteers, hospital workers or university students. Personal information was collected through questionnaire. Subjects were excluded from the study if there was any history of exercise discomfort, wheezing, occupational exposure to hazardous substances, asthma, allergic rhinitis, hay fever, urticaria, other allergic conditions or were on medications that might influence airway tone. If the subjects had any current cough, dyspnoea, sputum production, or any respiratory infection within two months, any cardiac disease or chest deformity then also they were excluded from the study. Written and informed consent was taken from each and every participant. The participants were instructed to avoid heavy physical activities, abstain from tobacco in any form and alcohol for at least 12 hours and tea or coffee for 2 hours before coming to the research lab and the subject was advised to come two hours after light breakfast. Each participant had 2 visits, first visit for general instruction about how to perform the test, so that they become accustomed with proper maneuver. The second visit involved the baseline and post exercise spirometry. The experiment was done in morning hours 9:00 AM to 12:00 noon to avoid circadian variations in the pulmonary function. Baseline PFT: Lung functions were measured by RMS Helios Series Computer based Spirometer with highly advanced and user-friendly software offering 34 parameter readings, Pre-Post bronchodialation results, Percentage Improvement and Lung Age Calculations. After giving rest for 15 minutes, baseline pulmonary function test was done using the Forced vital capacity (FVC) manoeuvre. Indices recorded were - Forced vital capacity (FVC), Forced expiratory volume in 1 second (FEV1), Mean forced expiratory flow between 25% and 75% of FVC (FEF25-75%), Mean forced expiratory flow between 75% and 85% of FVC (FEF75-85%). For baseline lung function three satisfactory manoeuvres were performed and best of three was chosen for calculation. Exercise challenge testing: For exercise challenge treadmill attached to 12 lead ECG monitor was used which continuously monitored heart rate and ECG while the subject was exercising. Participants were asked to exercise on the treadmill, so that they achieve 80-90% of the maximum predicted heart rate (220 - age) for 5 minutes. Whilst several protocols exist, we followed the most widely accepted protocol based on the Guidelines produced by the ATS in 1999. The guiding principle is to create a degree of hyperventilation by pushing the participants quickly to perform high cardiac output exercise5. This was done on the treadmill with the aim of achieving 80 – 90% of the maximal heart rate in 4 – 6 minutes. The heart rate was monitored through ECG and the test was stopped when the patient achieved 80 – 90% of the maximal heart rate. PFT was recorded immediately after completion of the exercise challenge followed by subsequent readings at 5, 10 and 20 minutes of recovery period. Data entry and Analysis: Data was entered in MS excel and analyzed using software MedcalC. Comparison of lung function before and after exercise in healthy volunteers and asymptomatic smokers respectively was done using paired t test.OBSERVATIONS AND RESULTS There was no significant difference in age, height, weight and baseline pulmonary function among the 48 volunteers who participated in the study including 24 healthy volunteers and 24 asymptomatic smokers (Table 1 and 2).
Table 1: General characteristics of the study group
Table 2: Baseline Pulmonary function before exercise challenge in healthy volunteers and Asymptomatic Smokers
Comparison of airway responsiveness to sub maximal exercise between asymptomatic cigarette smokers and healthy volunteers: - Fourteen of the 48 subjects taken had a ≥15% drop in FEV1 after sub maximal exercise challenge and they were grouped under category of responsive. Out of these 13 were smokers and 1 was non-smoker (Table 3). Table 3: Airway responsiveness to sub maximal exercise
Chi square= 14.521, p<0.001
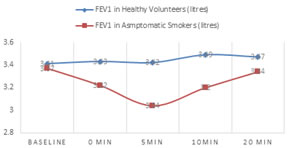
FEV1 as the measured variable The time pattern of changes indicating the response of the participants, the Mean±SD values for FEV1 at the baseline and after the exercise challenge at 0, 5, 10, 20 minutes of recovery period is shown in figure1, for both the groups. The values dropped from the baseline value (3.37±0.55) among asymptomatic smokers at 0 min (3.22±0.54), 5 minute (3.05±0.59), 10 minute (3.20±0.57), 20 minute (3.34±0.63) of recovery period, which was significant (p<0.05) at 5 and 10 minutes and highly significant (P=0.0001) taking one maximum change manoeuvre from the post exercise tests (Fig 2.2). In case of healthy volunteers values increased from the baseline (3.41±0.37) and was found significant (p<0.05) taking one maximum change manoeuvre from the post exercise recovery period.
Figure 1: Time pattern changes in FEV1 - baseline and after the exercise challenge at 0, 5, 10, 20 minutes of recovery period *t=2.32, p=0.02; **t=-4.802, p=0.0001
Forced Expiratory Flows: On comparing Forced expiratory flow rates before and after exercise amongst the healthy volunteers and the asymptomatic smokers respectively by paired t test, it is very clear that the flow rates on an average increased in case of non-smokers (table 4) and decreased in case of smokers (table 5).
Table 4: Comparison of Forced Expiratory Flow rates before and after exercise challenge in healthy volunteers
*p<0.05, t=2.66 In case of healthy volunteers the change was significant only in the most peripheral airways, FEF75-85% (1.3±0.51 Vs 1.46±0.54, p=0.01, t=2.66) while in case of the Asymptomatic Smokers the decline in flow rates was significant at absolute flow rates at 25% and 50% of FVC and also when taking an average flow rate for peripheral airways i.e. FEF25-75% as shown in table 5. Table 5: Comparison of Forced Expiratory Flow rates before and after exercise challenge in asymptomatic smokers
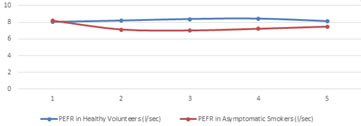
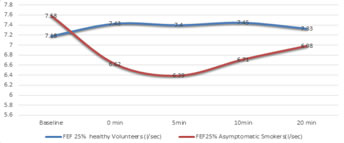
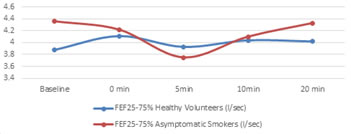
*p<0.05, t=-4.87; **p<0.05, t=-3.31; #p<0.05, t=3.2 Flow rates taken after exercise challenge followed a pattern same as FEV1 with time. There was a gradual increase in the flow rates of healthy volunteers almost immediately after exercise then started reducing to baseline again. In Asymptomatic smoker group there was a gradual decrease in flow rates in the recovery period first and then return towards the baseline again, as shown in figure 2.1 – 2.3, for PEFR,FEF 25%and FEF25-75% respectively. Except 3 all the asymptomatic smokers showed decline in their flow rates and 6 of the healthy volunteers had an average decrease instead of increase in flow rates.
Figure 2.1: Time pattern changes in PEFR - baseline and after the exercise challenge at 0, 5, 10, 20 minutes of recovery period
Figure 2.2: Time pattern changes in FEF 25% - baseline and after the exercise challenge at 0, 5, 10, 20 minutes of recovery period
Figure 2.3: Time pattern changes in FEF25-75% baseline and after the exercise challenge at 0, 5, 10, 20 minutes of recovery period
Comparison of percentage change of different pulmonary functions with exercise challenge: On comparing the average percentage change in both the groups after exercise (whose FEV1 was having no significant difference before exercise) turned out to be significantly different (p<0.0001), as shown in table 6.
Table 6: Mean percentage change in FES parameter in the post exercise state from the baseline values
For FEF25-75%, post exercise recovery values dropped significantly (p<0.001) from the baseline in Asymptomatic Smoker group (table 6). However, the values increased among the healthy volunteers. After exercise the values between the two groups, which were having insignificant difference before exercise, now were different significantly (4.36±1.19 Vs 3.72± 1.19, p=0.003, t=-3.204).
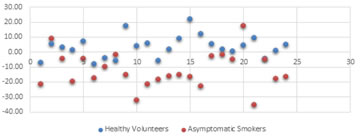
DISCUSSION Exercise is a remarkable example of the integrative and adaptive capacities of the body. Smoking may impose limitations on the capacity of the body to acquire, transport, and utilize oxygen in various ways. This limitation in aerobic capacity can be tested by calibrated exercise, either graded or global. In this study, we have compared changes in lung function before and after sub maximal exercise in healthy male volunteers and healthy male asymptomatic smokers in the age group of 18 to 25 years. Normal subjects show considerable airway dilatation during exercise and this appears to result from inhibition of resting vagal tone 6. Exercise mediated bronchodilation in normal individuals is most likely to occur in peripheral airways because they are more compliant. Through the assessment of the pulmonary function test following exercise challenge, the present study demonstrated that the asymptomatic smoker group with absolutely normal spirometry result at rest, had significant decrease in FEV1 and all other flow rates after exercise challenge. It seems to uncover the early underlying disease. Evaluating in term of a sensitive Forced Expiratory Spirogram (FES) parameter FEV1, 13 asymptomatic smokers out of 24, who were found responsive, had drop in FEV1 more than 15% within 20 minutes of post exercise recovery period (table 3). It shows that bronchodilatation response to exercise is weak in these smokers which may be because of the associated heightened pathophysiology of inflammation of airways. Similar results were seen in a study done in Nepal by Pokhrel et al.. where they found 22 responsive smokers out of 42 asymptomatic smokers (4). However, they have not compared healthy non-smokers with the smokers for hyper-responsiveness to the modality of challenge used. Another study in the asymptomatic smokers by Quaedvlieg et al.. also has demonstrated the Broncho-constrictive response to cold air challenge which is extending largely into the small peripheral airways by impedance measurement method which was not seen in normal subjects7. In few early studies bronchial reactivity was not demonstrably greater in younger smokers than in non-smokers less than 36 years old (which used higher concentrations of histamine). Several other investigations of young symptomless smokers with normal lung function have also failed to detect any consistent difference in bronchial reactivity though one report found slightly diminished reactivity.(8) On the contrary, our study which focused on young asymptomatic smokers (18 to 25 years) found significant number of young early smokers (13 out of 24) to having bronchial reactivity and the rest non-responsive also showed some decline in the peripheral airway function after challenge at p<0.001 level of significance when compared with the normal healthy volunteers (Table 3). As shown in fig.1, most of the asymptomatic smokers had a decrease in FEV1 from the baseline after exercise challenge (shown as red spots in the scatter diagram) while most of the healthy non- smokers had a increase in FEV1 after exercise challenge (shown as blue spots). The difference of results in studies may have been due to the difference in type of bronco-provocation used. Indirect challenges act by causing the release of endogenous mediators that cause the airway smooth muscle to contract, with or without effect in inducing microvascular leakage. Because the responses to these challenges are modified or even completely inhibited by inhaled steroids, the airway response to these challenges may be a closer reflection of active airway inflammation. 9 In the current study, a clear trend of decline in FEV1 in smokers with comparable history and nicotine dependence ascertained by Fagerstrom Test for Nicotine Dependence questionnaire, may emphasize that exercise challenge testing might be useful tool for screening mechanical abnormalities induced by smoke inhalation even before there is any change in pulmonary function at rest. Different processes might be responsible for the changes in airway function during and after in both modes of the challenges, but the post exercise decline in FEV1 was the pertinent fact in both of the cases. There are ample evidences available which have shown a broncho-constrictive response to various nonspecific stimuli including exercise challenge and airway hyper responsiveness to provocative agents in asymptomatic smokers having an alteration of ventilatory functions due to hyper reactive peripheral airways.(10) The pathological changes found in smoker’s lung which could be responsible for active muscle constriction and airway narrowing following the challenges may include: a) airway epithelial damage, resulting in increased permeability and impairment of other epithelial functions; b) chronic airway inflammation; c) structural changes in the airway wall; and d) loss of alveolar attachments. However, one or more than one processes might be at work in smokers or might be not all smokers develop the above mentioned airway abnormalities (11). The effects of smoking on small or peripheral airways have been addressed by many studies in asymptomatic smokers (12)(13). However, the effect of exercise challenge on peripheral airway mechanics in asymptomatic smokers is the novel part of our research. The airway dilatation and thus decrease in airway resistance is a common physiological response to exercise for normal people, but it appears that partial airway obstruction and weak elastic recoil of lungs is evident in smokers which is leading to a subclinical alteration of ventilatory patterns of small or peripheral airways due to active cigarette smoking. Hyperventilation during the exercise causes drying of the airways, which increases the osmolarity of fluid lining the airways. This provides an osmotic stimulus for water to maneuver from any cell nearby, leading to cell volume loss (14). The change in regulatory volume after cell shrinkage is believed to be the factor causing release of inflammatory mediators, which then cause the contraction of airway smooth muscles resulting in narrowing of airways. In-vitro studies of human lung mast cells show that increased osmolarity of the solution bathing the cells is a potent stimulus for release of histamine. J A Dosman et al., who evaluated peripheral airways in middle age heavy smokers by helium flow-volume curves and single breath nitrogen test, also indicate that the individuals who responded to inhaled histamine had increase in airways reactivity and peripheral airways dysfunction both. Finnerty J and Holgate S in their study have supported role for histamine release by the finding that some histamine H1 receptor antagonists have an inhibitory effect on exercise induced bronchoconstriction (EIB) 15. Leukotrienes are involved in the genesis of EIB and in sustaining the bronchoconstriction following exercise. Reiss T F et al.. have reported increases in urinary leukotriene E4 following EIB. Extensive mast cell infiltration in asymptomatic smokers at all levels bronchial mucosa predominantly in the airway smooth muscle compartment has been reported by A Ekberg-Jansson et al..16. Smoke exposure has been shown to induce activation/ degranulation in airway resident mast cells and elevated levels of histamine and tryptase have been measured in the bronchoalveolar lavage fluid of active smokers. Mast cell-secreted proteinases (MCPTs), including tryptases, have been considered to be the contributors of pathological airway remodelling. Andrea Small-Howard and Helen Turner in their study concluded that chronic exposure to cigarette smoke up-regulates MCPT levels in mast cells at both the protein and the mRNA level. They suggested that the pathological airway remodelling that has been described in clinical studies of smoke inhalation may be attributable to MCPT overproduction in vivo 17. Thus, we can say that by using exercise as a bronchoprovocation test we are actually getting the warning of the ignition factor of pathological changes for COPD in future. Exercise being a non-pharmacological and non-invasive tool can be easily used for screening the otherwise healthy smokers with normal spirometry. All these changes which are a deviation from normal airway function may go un-noticed if pulmonary function test parameters at rest are only considered (18). Although, cigarette smoke causes the airway hyper responsiveness which is a primary risk factor for development of COPD (chronic obstructive pulmonary disease) or chronic bronchitis, most of the smokers do not develop symptomatic COPD, probably because of the large physiological reserve of the lung. Interestingly, around 15- 20% do experience accelerated loss of lung function, and the basis for heightened susceptibility in the smokers is yet to be known 19. In our study flow rates at rest were a little higher in smokers as compared to the non-smokers but after exercise challenge there was a decline in smokers while an increment in non-smokers. This also makes it clear that the negative reaction in smokers was not merely due to morphometric difference in the airways at rest as suggested by Taylor R et al. and Brusasco V et al.. 8,20. A more specific effect of smoking, which is apparent within a few days of the starting of smoking, is increase in airway permeability, as shown by the rapid removal from the lungs of radiolabelled diethylenetriamine penta-acetic acid (DTPA) aerosol by Taylor R et al. Increased permeability of the airways, however, cannot account entirely for the abnormal bronchial reactivity of smokers. Probability of increase in bronchial reactivity during the smoking years by an immunological mechanism is more likely8. Observation of our study supports the point and suggests that the so called healthy smokers who have not been smoking cigarettes for long and seem to have good lung function at rest, are actually in the early phase of cigarette smoke induced mechanical deformity of airways.
Increment was seen in all parameters of Forced expiratory spirogram (FES) after the exercise challenge in healthy volunteers which was distinct in the most peripheral airways while in smokers there was a significant decrease in all parameters of FES. In our study 54% of asymptomatic smokers were found to have hyper-responsiveness, when they were subjected to exercise challenge as an indirect bronchoprovocation test. It shows that bronchodilation response to exercise is weak in these smokers which may be because of associated inflammation of airways. Using the indirect bronchoprovocation test like exercise we can uncover abnormal functioning of airways before any permanent damage to the airways is done by smoke in the so called ‘healthy smokers’ and reports deviating from normality may also motivate smokers to quit smoking early. On the contrary, spirometry at rest may not detect the early smoking induced changes in the airways and will give a false assurance that airway functions are normal. Exercise being a non-pharmacological and non-invasive tool can become an integral part in identifying early obstructive airway disease in future. Early detection of smoking induced changes in airway function and timely intervention may prevent the development of lifelong disability due to COPD. A combination of pharmacological and non-pharmacological test can give more elaborated results though but exercise induced are good tool for screening purposes. Further in-depth study by correlating it with molecular level histamine release and histological changes in bronchial wall at small airways can be planned.
Acknowledgements The authors are thankful for the active participation of all the study participants. We are also thankful to our teachers who guided us throughout the study.
REFERENCES
Policy for Articles with Open Access
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 Home
Home