|
Table of Content - Volume 15 Issue 2 - August 2020
Evaluation of thyroid function status in polycystic ovary syndrome
M Shareefa1, Syeda Sobia Harmain2*, Aruna BMK3
1Assistant Professor, 2P.G Student, 3Professor & HOD, Department of Physiology, Kakatiya Medical College, Warangal, Telangana, INDIA.
Abstract Background: Polycystic ovary syndrome (PCOS) is the most common endocrinopathy seen in reproductive age women. Recent literature suggests an increase in the incidence of thyroid disorders in females with polycystic ovary syndrome. The aim of this study is to evaluate the occurrence of thyroid dysfunction in subjects with polycystic ovarian syndrome as compared to controls of same age group. The study was a case control study conducted on 90 women, in whom 60 women were diagnosed with PCOS euthyroid and PCOS hypothyroid each and 30 normal healthy women with regular menstrual cycles as the control group within the age group of 20 to 40 years. The study was conducted at the department of Physiology, Kakatiya medical college, Warangal. The study revealed that the thyroid dysfunction was observed in the PCOS groups when compared to the control group. The PCOS hypothyroid women had significantly increased levels of TSH levels when compared to the PCOS euthyroid and control women. The T3 levels were significantly reduced in the PCOS hypothyroid women when compared to PCOS euthyroid and control groups. T4 levels were similar in all the groups. The FSH levels did not show any significant difference between the PCOS euthyroid and PCOS hypothyroid groups, but LH levels showed statistically significant difference between the PCOS euthyroid and PCOS hypothyroid groups. There was an association between the PCOS and the thyroid dysfunction as the TSH levels of the PCOS euthyroid were significantly higher than the control groups and very high in the PCOS hypothyroid groups. Key Words: Euthyroid, FSH, Hypothyroidism, Polycystic ovarian syndrome, LH
INTRODUCTION Polycystic ovarian syndrome (PCOS) was first reported by Stein and Levebthal in 19351. It is a most common endocrine disorder in the reproductive age group women. PCOS is characterized by menstrual irregularities and endocrine irregularities which results in the anovulation, infertility, and hyperandrogenism. The most common endocrine irregularities seen in PCOS are Insuline resistance (IR) and hyper androgenism. The prevalence of PCOS in the reproductive age women was 8 to 12% according to the Rotterdam criteria and recently the prevalence is raised to 18 % 2, 3. Thyroid disorders are the most common endocrine disorder globally. The etiopathogenesis of the PCOS and the hypothyroidism are completely different but are associated with menstrual disturbances’ and infertility4, 5. Thyroid disorders are commonly observed in PCOS subjects especially hypothyroidism, in which the thyrotropin releasing hormone (TRH) raises and leads to altered follicle stimulating hormone (FSH)/luteinizing hormone (LH) ratio and raised dehydroepiandrosterone (DHEAS) levels. Raised thyroid stimulating hormone (TSH) leads to stimulation of FSH receptors.6 Thyroid hormonal changes cause cystic ovarian changes with raised ovarian mass7. Few studies reported that the thyroid disorders are more common in women with PCOS when compared to the normal population 8-10. This may be due to some common factors predisposing an individual to both disorders or may be due to unestablished pathophysiological connection between the two disorders. There is limited data available regarding the prevalence of hypothyroidism and thyroid dysfunction in patients with PCOS especially in South India. Hence, the present study was aimed to evaluate the occurrence of thyroid dysfunction in subjects with polycystic ovarian syndrome as compared to controls of same age group. The aim of this study is to evaluate the occurrence of thyroid dysfunction in subjects with polycystic ovarian syndrome as compared to controls of same age group.
MATERIALS AND METHODS The present study was a case control study conducted on 90 women in which, 60 women were diagnosed with PCOS and 30 normal healthy women with regular menstrual cycles as the control group within the age group of 20 to 40 years. The study was carried out from January 2018 to May 2019 (15 months). The study was conducted at the department of Physiology, Kakatiya medical college, Warangal in collaboration with Obstetrics, gynaecology, infertility clinics at Kakatiya medical college/ hospital, Warangal. Inclusion criteria: The subjects who were already diagnosed (by Rotterdam criteria) with PCOS as case group and normal healthy subjects with regular menstrual cycle as control group who were within the age group of 20 – 40 years were included in the study. Exclusion criteria: The subjects with known history of any systemic diseases which affects the hormones, subjects who are on medication which may affect the hormones, the subjects who were on the oral contraceptive pills, the pregnant women, lactating women and also the women with the recent history of abortion were excluded from the study. The subjects were recruited after obtaining a complete medical and surgical history including menstrual history, the age of onset of menarche, family history of PCOS, and history of hirsutism, acne, alopecia, infertility and also about the history of last pregnancy and/or abortion. Any histories of headaches or blurred vision any signs or symptoms of thyroid dysfunction including acne, hirsutism, deepening of voice, and increase in muscle mass were also recorded. The subjects present age and the age of attainment of menarche were noted in all the subjects. The height, weight were measured and the body mass index (BMI) was calculated and recorded. In the present study the subjects were categorized into 3 groups. All the subjects were observed investigated for serum thyroid stimulating hormone (TSH) and T3 and T4 levels. The normal TSH level was considered as 0.5-4.5 mIU/L and the normal freeT3 and T4 levels were considered as 2.4-4.2 ng/ml and 0.7-1.24 ng/dl respectively. If thyroid is in normal level, those are included in control group and PCOS subjects were again classified into 2 groups based on thyroid hormone levels as euthyroid and PCOS hypothyroid.
Table 1: Showing the distribution of sample in the study
About 4 ml of fasting venous blood was collected in aseptic conditions from all the subjects into serum vacutainer (Red cap) for thyroid profile. The samples were stored in refrigerator at 2 to 80C. The samples were centrifuged and the serum was separated. The thyroid stimulating hormone (TSH), triiodothyronine T3, thyroxine T4, was estimated by using Chemiluminescence Immunoassay (CLIA) using Siemens adiva centaur XPT immunoassay analyzer, follicle stimulating hormone (FSH) were measured by using automatic Beckman Access 2 Immunoassay System and LH Hormone measured by enzyme-linked immunosorbent assay. Statistical analysis The range, mean and standard deviations were calculated for age, height, weight, BMI, age at menarche, T3, T4, TSH and FSH hormones. The data was also processed by using ‘t’ test to find out the level of significance between the groups at 95% CI.
OBSERVATIONS AND RESULTS This study includes normal healthy subjects in control group and PCOS subjects in euthyroid and hypothyroid groups. This study is conducted to evaluate the thyroid hormone status along with levels of the FSH, LH and the LH/FSH ratio in the PCOS Euthyroid and PCOS Hypothyroid when compared with healthy controls. The average age at menarche of the control group was 12.03±0.96 years with the range of 11 to 14 years, of PCOS euthyroid group was 12.03 ± 0.80 years with the range of 11 to 14 years, of the PCOS hypothyroidism group was 12 ± 0.83 years with the range of 11 to 14 years. The means of height, weight, BMI, TSH, T3, T4, FSH and LH hormones of the control group, PCOS euthyroid group, PCOS hypothyroidism group were recorded in table 2. The data was processed using ‘t’ test and the level of significance was considered as P < 0.05
Table 2: Comparison of the hormone levels in all the groups
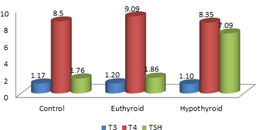
The T3 levels were statistically significantly lower in the control group and PCOS euthyroid groups when compared to the PCOS hypothyroid group. There was no significant difference between the control and PCOS euthyroid group. Significant decrease in the T3 levels was observed in the PCOS hypothyroid group when compared to PCOS euthyroid group (P= 0.03) and control group (P= 03). The T4 levels were observed to be similar in all the groups. There was no statistically significant difference among the 3 groups. The TSH levels were observed to be significantly higher in the PCOS hypothyroid group when compared to the control and the PCOS euthyroid groups.
Figure 1: Comparison of the thyroid hormone levels in all the groups
The FSH levels did not show any significant difference between the PCOS euthyroid and PCOS hypothyroid groups (p=0.46) The mean LH in the PCOS euthyroid, and PCOS hypothyroid groups were 7.93± 4.2mlU/ml and 10.98± 4.63mlU/ml respectively. Statistically significantly higher levels were observed in PCOS hypothyroid group when compared to PCOS euthyroid group with p value of 0.0049.
Figure 2: Comparison of the FSHandLH hormone levels in all the groups
DISCUSSION Recent literature suggests an increase in the incidence of thyroid disorders in females with polycystic ovary syndrome. A study by Sinha et al.,[8] compared 80 PCOS women with 80 controls and reported significant increase in the prevalence of goiter and subclinical hypothyroidism in PCOS women when compared to the normal control groups. Another study reported that, the prevalence of PCOS women with subclinical hypothyroidism as 11.3%11. In this present study the thyroid function was assessed by measuring the serum thyroid hormone levels in all the 3 groups. The mean age of the women in control group was 30.56±3.15 years, in the PCOS euthyroid group was 30.33± 3.29 years and in the PCOS hypothyroid group was 26.26 ± 3.92 years. The mean age is less in the PCOS hypothyroid group as they present early due to menstrual irregularities caused by both hypothyroidism and PCOS. In a study by Kedar et al., 7 found the mean age of the women in PCOS and control groups were 22.86±3.5 years and 24.3±3.5 years respectively and found that, the mean age is less in the PCOS group compared to control group as they present early due to menstrual irregularities. The BMI of the PCOS hypothyroid women shows significantly higher values when compared to control groups with (P = 0.013). These results are similar to the others results7,12 Over weight and obesity are commonly observed in both PCOS and hypothyroidism, so there was a significant increase in the BMI in PCOS and hypothyroid groups13. In the present study the mean TSH levels in control, PCOS euthyroid and PCOS hypothyroid groups were 1.76±0.81µU/ml, 1.86±0.65µU/ml and 7.09±2.68µU/ml respectively. The PCOS euthyroid group values were within the normal range but significantly higher than the control group where the p value was 0.001 and the PCOS hypothyroid group was very high compared to the control and the PCOS euthyroid groups. Many studies have been done to find out the association between the TSH and PCOS, but few studies reported an association between the control and PCOS groups as the TSH values were significantly higher in PCOS group compared to control groups8,12,13. In the present study, the PCOS subjects were divided into 2 groups based on the TSH levels into PCOS euthyroid group and PCOS hypothyroid group and compared the thyroid profile between all there 3 groups and found statistically significant difference between them. In the present study, in all the subjects T3 and T4 were also estimated along with TSH and the levels were compared between the groups. The mean T3 levels in the control, PCOS euthyroid and PCOS hypothyroid groups were 1.17ng/dl, 1.20ng/dl and 1.10ng/dl respectively. There was a marked decrease in the T3 levels of PCOS hypothyroid group when compared to PCOS euthyroid and control groups these results were in similar with other study by Kedar et al.7. As these two studies were done in the Indian population, there was not ethnic variation also, which would be a factor to find the similar results. In the present study the mean T4 levels in the control, PCOS euthyroid and PCOS hypothyroid groups were 8.5ng/dl, 9.091ng/dl and 8.35ng/dl respectively. The T4 levels were similar in all the groups and there was no significant difference found among the groups which is coinciding with other studies by Kedar et al..7, and Elslimani et al.14, but in contrast with the study by Eldin et al.15 where they had reported a significant decrease in the T4 levels (P < 0.01) of the PCOS group when compared to control group and they also found difference in the T4 levels between the PCOS lean and PCOS over weight groups. According to Carvalho et al..16 and Schussler et al.,17any alterations in the transporter proteins may give rise to altered levels of total T4 levels irrespective of its thyroid status. Thus the lower T4 cannot be explained by isolated thyroid abnormality. There was no significant difference between the levels of FSH in PCOS euthyroid and PCOS hypothyroid groups. The present study results were nearer to the other study by Ramanand SS et al.,[18] who reported the mean FSH levels of PCOS euthyroid and PCOS hypothyroid as 5.70 ± 1.80mlU/ml and 5.32 ± 1.54mlU/ml respectively. The mean LH levels in PCOS euthyroid and PCOS hypothyroid groups were 7.93mIU/ml and 10.98 mIU/ml respectively. Statistically significant difference was observed between the PCOS euthyroid and PCOS hypothyroid groups (p=0.004) which is similar to the study by Ramanand et al.,[18] who reported the average LH levels in PCOS euthyroid and PCOS hypothyroid as 12.54mlU/ml and 10.38mlU/ml respectively. The LH/FSH ratio did not show any significant difference between the PCOS euthyroid and PCOS hypothyroid groups.
CONCLUSION The present study revealed that the thyroid dysfunction was observed in the PCOS groups when compared to the control group. The PCOS hypothyroid women had significantly increased levels of TSH levels when compared to the PCOS euthyroid and control women. The T3 levels were significantly reduced in the PCOS hypothyroid women when compared to PCOS euthyroid and control groups. T4 levels were similar in all the groups. The FSH levels did not show any significant difference between the PCOS euthyroid and PCOS hypothyroid groups, but LH levels showed statistically significant difference between the PCOS euthyroid and PCOS hypothyroid groups. There was an association between the PCOS and the thyroid dysfunction as the TSH levels of the PCOS euthyroid were significantly higher than the control groups and very high in the PCOS hypothyroid groups. Thus, the physicians should routinely advice the thyroid profile for the PCOS patients and treat the thyroid dysfunction which may help in the management of menstrual irregularities and infertility.
ACKNOWLEDGMENTS The authors are thankful to the Kakatiya medical college/Hospital for providing facilities to conduct this study.
REFERENCES
Policy for Articles with Open Access
|
|
 Home
Home