|
Table of Content - Volume 19 Issue 2 - August 2021
Effect of single bout of exercise on heart rate variability in young healthy adults and its correlations with anthropometric parameters
Deepayan Sarkar1, Anindya Roy2, Paramita Nag3, Gargi Barat4, Enakshi Ghosh5*
{1Junior Resident (3rd year), Department of Ophthalmology} {2Associate Professor and HOD, Department of Physiology} Raiganj Government Medical College, Raiganj, Uttar Dinajpur, West Bengal, INDIA. 3Demonstrator, 4Junior Resident IInd Year, 5Associate Professor, Department of Anatomy, R.G. Kar Medical College, Kolkata, INDIA. Email: drenakshighosh@gmail.com
Abstract Background: Heart rate variability (HRV) as a measurement of autonomic function assumes great clinical importance. It is well known that particular patterns of body fat distribution increase coronary heart disease risk both in adults and children. While frank obesity is associated with reduced HRV, indicative of poorer autonomic nervous system (ANS) function, the association between body mass index (BMI) and HRV is less clear. The dynamic autonomic responses during exercise can be measured to give actionable information for training by analysis of the ECG to determine heart rate variability. While application of HRV has been applied to predict sudden cardiac death and diabetic neuropathy in assessing disease progression. The study revealed the changes in HRV in resting condition and also after a single bout of sub maximal treadmill exercise (50% of VO2 max.) among males and females in the age group of 17-25 years at rest, and the correlations between the HRV parameters at rest and after the exercise with BMI and WHR in the subjects. An observational cross sectional study was conducted in the Physiology Department of R.G.Kar Medical College, Kolkata, India on 60 subjects (n=60) of both sexes (30 males and 30 females) in the 17-25 years age, participated in this study. Their WHR and BMI were measured and HRV was recorded during rest and immediately after exercise by digital Polyrite. Result showed that the HRV of male were more than in female in resting condition. After submaximal exercise the HRV value of males were more than their respective resting HRV values though it was not significant and, in the females, post-exercise HRV was significantly more than their respective resting HRV. This study shows that females have higher parasympathetic activity than males. There is an association between WHR and BMI and HRV in healthy female persons, which shows that there is an increase in LF/HF(low frequency and high frequency) of values among males and females after a single bout of sub-maximal exercise though not significant in case of males but significant in case of females. Keywords: Heart Rate Variability, Waist Hip Ratio, Basal Metabolic Rate, LF/HF Ratio, Submaximal, Autonomic Nervous System, Exercise, Digital Polyrite.
INTRODUCTION The Autonomic nervous system (ANS) is the part of the Central nervous system (CNS) that acts as a control system that functions largely below the level of consciousness to control visceral functions, including heart rate, digestion, respiratory rate, salivation, perspiration, pupillary dilation, micturition, sexual arousal, breathing and swallowing. Most autonomic functions are involuntary but they often work in conjunction with the somatic nervous system which provides voluntary control.1The autonomic nervous system is controlled mainly by centers located in the spinal cord, brain stem, hypothalamus and also the portions of the limbic cortex of the brain. The medulla which is a part of the brainstem has the cardiac regulatory centre. The efferent autonomic signals are transmitted to the various organs of the body through two major subdivisions called the Sympathetic Nervous system(SNS) and the Parasympathetic Nervous system(PNS). The sympathetic nerve fibers originate in the spinal cord segments T1-L2 and pass into the sympathetic chain and then into the tissues. The parasympathetic nerve fibers leave the central nervous system through cranial nerves III, VII, IX and X, about 75% of the fibers are in the vagus nerve(X).2The effect of autonomic nervous system on heart can be summarised as:
Heart rate variability (HRV) is the physiological phenomenon of variation in time interval between heart beats. Variation in the beat-to-beat interval is a physiological phenomenon4.HRV has an ubiquitous relationship with all biological functions of the body. The HRV data can be collected by non-invasive procedures and is subject friendly. Presently, it is studied widely in India and abroad, its clinical implications are also emerging gradually. The SA node receives several different inputs and the R-R interval and its variation are the results of these inputs. The main inputs are from the sympathetic and the parasympathetic nervous system5.Most widely used methods of HRV analysis are Time Domain method, Frequency Domain method, Non-linear method, Geometric method. In this study we will study HRV by Frequency Domain Method. This method is chosen as, by this method in stable standardized conditions no other factor other than the current autonomic tone contributes in HRV.6So, by calculating HRV by FFT method we can measure accurately the effect of persisting autonomic tone on the heart. The Frequency domain is calculated by Fast Fourier Transformation (FFT). The frequency domain in short term recording has 3 components- Very low Frequency (VLF), Low Frequency (LF), High Frequency (HF). Of these, the physiological aspect of VLF is less defined. Sympathetic tone is believed to influence the LF component whereas both sympathetic and parasympathetic activity has an effect on the HF component. The heart rate is increased by sympathetic activity and decreased by parasympathetic (vagal) activity. The balance between the effects of the sympathetic and parasympathetic systems is called sympathovagal balance and it reflects in the beat-to-beat changes of the cardiac cycle. The ratio of the power (LF/HF) is used as a measure of sympathovagal balance. 7 Obesity is a clinical condition in which excess body fat has accumulated to that extent that it has negative impact on health. Excessive body weight is associated with diseases like cardiovascular diseases, diabetes mellitus type 2, obstructive sleep apnoea, cancers etc. In the cardiovascular system it causes diseases like ischemic heart disease, congestive heart failure, high blood pressure.8 Obesity is measured by particular anthropometric parameters, though body mass index (BMI) is the standard measure of obesity, it is seen that waist hip ratio (WHR) is well correlated with the clinical implications of obesity and abnormal fat deposition in the body.9 Recent studies also prove that the WHR is a better indicator of cardiac status than the BMI.10 In this study both the parameters BMI and WHR are taken for anthropometric correlation. This study shows the effect of obesity on ANS which is evident by HRV analysis. Sex of a person by itself is an independent variable that is seen to affect the HRV of an individual.11 It is seen in females that a considerable change in HRV is noted during the different phases of the menstrual cycle, this change is due to the effect of different sex hormones attaining their peaks in different phases.12 These hormones lay their effect on the heart rate and HRV of an individual. Present study aims to discover if any significant difference in HRV persists among males and females in resting condition and after submaximal exercise. The subjects were given a sub maximal graded exercise in which the intensity of exercise gradually increases. The exercise was given according to the modified Blake-Bruce Protocol.13 A treadmill exercise was given to the subject until. was reached. This was measured by a pulse oxymeter. When the pulse rate reaches 128 beats/min (for males) and 138 beats/min(for females) it indicates 50% of VO2max. The exercise was then terminated. The subject was then immediately laid in supine condition to record the HRV. The last two decades has witnessed the recognition of a significant relationship between the ANS and cardiovascular mortality, including sudden cardiac death. Experimental evidence for an association between diseases of cardiovascular system (cardiac autoneuropathy) either increases the sympathetic or reduces the vagal activity this has spurred the efforts for the development of quantitative markers for autonomic activity.15 Reduced HRV and heart rate has been shown to be a predictor of mortality after acutemyocardial infarction(AMI).16,17,18A range of other conditions are also associated with HRV, including congestive heart failure, diabetic neuropathy, depression, post-cardiac transplant, susceptibility to SIDS and poor survival in premature babies. The purpose of this study is to find out if a single bout of sub-maximal exercise (50% VO2 max.)can cause change in HRV and how it varies with gender and anthropometric parameters. This study will show how gender and obesity influences the HRV which is a measure of autonomic nervous system. This study also lays clinical standards of the HRV in correlation with obesity and sex hormones which would effectively help a physician to diagnose and treat an obese patient or a patient undergoing hormone replacement therapy(HRT).
AIMS AND OBJECTIVES
METHODOLOGY
the basal heart rate. G. Exclusion Criteria:
H. Instruments Used
I. Data collection procedure: 1. Questionnaire: Includes History, General Examination and Systemic Examinations. 2. Investigations:
J. Statistical Tools: Statistical analysis was done by SPSS version -17 using Unpaired student T-Test, Paired Student T- test and Pearson Correlation Test. P value <0.05 was taken as significant value. K. Ethical Consideration: Ethical clearance has been taken from the ‘Ethics Committee’ of R.G Kar Medical College and Hospital, Kolkata. L.Written Consent: Written consent was taken from all subjects taking part in this study after proper explanation of the study, procedures and its impacts. M. Measurement of HRV: a) Pre-study criteria: A modified questionnaire (Mayo Autonomic Laboratory, Mayo Clinic, Rochester, Minnesota)26 containing subject particulars, personal habits, family history and a detailed history of any illness or treatment were recorded. General physical examinations were done. Pre-test instructions were given to avoid consumption of any drugs or food that may alter the autonomic function, 48 hours prior to the test. The subjects were advised to have sound sleep the night before. On the day of the test subjects were not permitted to have tea, coffee, food or drugs from three hours prior to the tests. b) Procedure:
c) Measurement of WHR and BMI: i. Waist Circumference- It is the point midway between lower rib and iliac crest i.e., 1cm below theumbilicus. ii. Hip Circumference- It is measured as the widest girth of the hip. iii. BMI measurement: It was measured by Quitlet index i e. Weight in kg / (Height in Metre)2 WHR: i. Normal= 0.80-0.87(males) , 0.67-0.75(females). ii. Overweight = 0.88-0.99(males), 0.76-0.84(females). iii. Obese = ≥1(males), ≥0.85(females). BMI- (kg/m2): i.<18.50 (underweight) ii.18.50-24.99 (normal) iii.25-29.99 (overweight) iv.≥30.00 (obese).27 d) Analysis of the recordings: In frequency domain [in short term recordings (2-5 minutes)], spectral components are:
The physiological component of VLF is much less defined. The measurement of VLF, LF, and HF power components was be taken in absolute values of power (milliseconds squared). LF and HF values were also measured in normalized units, which represent the relative value of each power component in proportion to the total power minus the VLF component. The representation of LF and HF in normalized units emphasizes the controlled and balanced behaviour of the two subdivisions of the ANS.28The data of LF/HF ratio was collected and tabulated using suitable statistical techniques and was then analysed to find the correlations, if any.
OBSERVATIONS AND RESULTS Table 1: Gender wise distribution of subjects
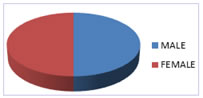
Figure 1: The pie diagram shows the distribution of study subjects according to gender.
Table 1 and Fig.1 shows that: 50% of the subjects taken for the study were males and 50% were females.
Table 2: Age wise distribution of subjects
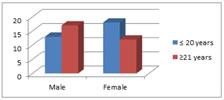
Figure 2: The bar diagram shows the distribution of subjects according to gender and age. Table 2 and Fig. 2 shows that:Among the 30 male.Among the 30 female subjects taken 18 were of the age group 17-20 (≤20) years and 12 were of the age subjects taken 13 were of the age group 17-20 (≤20) years and 17 were of the age group of 21-25 (21) years group of 21-25 (21) years. Table 3: Base line charecteristics of the subjects
*p<0.05 = Significant. Table 3 shows that male and female were age matched. There was no significant differences in BMI,WHR and pulse rate except height and weight that was found to be significantly (p<0.05) more in males than females. Table 4: Comparision of BMI with Gender of the subjects
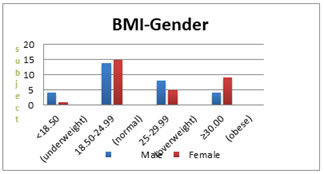
Figure 3: The bar diagram shows the distribution of BMI with gender of the subjects.
Table 4 and Fig. 3 shows that among the study subjects maximum number of subjects were normal (46.67% males and 50% females)where as only 13.33% males and 3.33% female were underweight, 26.67% males and 16.67% females were overweight and 13.33% males and 30% females were obese.
Table 5: Comparison ofWaist-Hip Ratio (WHR) with Gender
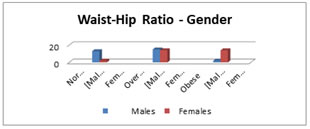
Figure 4: The bar diagram shows the distribution of study subjects acoording to WHR and Gender. Table 5 and Fig.4 shows that among the female only 6.67%were normal but 46.67% were obese and 46.67% were overweight. Whereas among the male maximum number of subjects were overweight (50%) , 6.67% males were obese. Only 43.3% males were in normal category. Table 6: Comparision of LF/HF Ratio with gender after sub-maximal exercise
*p<0.05 = Significant. Table 6 shows that there is a significant difference of resting LF/HF values among males and females.After a single bout of sub-maximal exercise there is no significant change in LF/HF values in males,but there is significant change in LF/HF values in females after the exercise. There is no significant change in LF/HF ratio among males and females after the exercise. TABLE 7: Correlation of BMI and LF/HF ratio (HRV parameters) for Pre and Post Exercise in Males
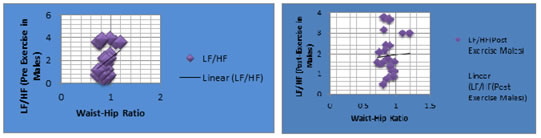
Figure 5: The scatter diagram shows the correlation between pre-exercise LF/HF ratio with BMI among males; Figure 6: The scatter diagram shows the correlation between post-exercise LF/HF ratio with BMI among males Table 7 and scatter-diagrams (Fig.5 and Fig. 6 ) shows that Pre- Exercise LF/HF ratio in Males has weak positive correlation (r=0.251)with BMI and Post Exercise LF/HF ratio in Males has weak negative correlation (r=-0.023) with BMI and that correlations were not significant (p >0.05). Table 8: Correlation of WHR and LF/HF ratio (HRV parameters) for Pre and Post Exercise in Males
Figure 7: The scatter diagram shows the correlation between pre-exercise LF/HF ratio with WHR among males.Figure 8:The scatter diagram shows the correlation between post-exercise LF/HF ratio with WHR among males.
Table 8 scatter diagrams (Fig.7 and Fig. 8) shows that Pre- Exercise LF/HF ratio in Males has positive correlation with WHR and Post- Exercise LF/HF ratio in Males has weak positive correlation with WHR but that was not significant. TABLE 9:Correlation of BMI and LF/HF ratio (HRV parameters) for Pre and Post Exercise in Females
*p<0.05 = Significant.
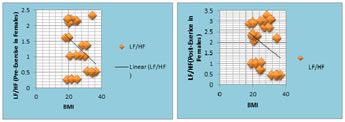
Figure 9: The scatter diagram shows the correlation between pre-exercise LF/HF ratio with BMI among females; Figure 10: The scatter diagram shows the correlation between post-exercise LF/HF ratio with BMI among females.
Table 9, Fig.9, Fig.10 shows that Pre-Exercise LF/HF ratio in Females has negative correlation with BMI and that is significant whereas Post-Exercise LF/HF ratio in Females has negative correlation with BMI but that is not significant.
TABLE 10: Correlation of WHR and LF/HF ratio (HRV parameters) for Pre and Post Exercise in Females
*p<0.05 = Significant.
Figure 11: The scatter diagram shows the correlation between pre-exercise LF/HF ratio with WHR among females; Figure 12: The scatter diagram shows the correlation between post-exercise LF/HF ratio with WHR among females.
Table 10, Fig.11 and Fig.12 shows that Pre Exercise LF/HF ratio in Females has significant association with WHR but Post Exercise LF/HF ratio in Females has Significant negative correlation with WHR.
DISCUSSION The HRV is due to autonomic modulation over the cardiac activity and it is well known that the decrease in HRV indicates sympathetic activity and increase in HRV is due to parasympathetic influence. So, decrease in LF/HF indicates decrease in sympathetic activity and increase in LF/HF indicates increase in parasympathetic activity. Deep breathing activates parasympathetic nervous system whereas during exercise the sympathetic activity is kindled. In the present study, the basal HRV (resting period) of males are significantly more than females indicating sympathetic activity is more in male than female. According to Kuo et al.’s study middle-aged females have a more dominant parasympathetic regulation.29The reason for this is paradoxical autonomic control in adolescent age, lies in the onset of secretion of sex hormones. The female sex hormones play a vital role in increasing the vagal activity. This increases the parasympathetic activity in young adolescent girls. This is also shown in the study conducted by A. S. Leicht, et al., in which it was shown that there is an increased vagal tone in girls during ovulation and he concluded that it was due to increased endogenous oestrogen level.30As there is an increased level of sex hormones especially oestrogen during the adoloscence, this causes an increased parasympathetic activity by increasing the vagal tone which maintains a low resting LF/HF ratio in females. Physical exercise is associated with parasympathetic withdrawal and increased sympathetic activity resulting in heart rate increase. The rate of post-exercise cardio deceleration is used as an index of cardiac vagal reactivation. Analysis of HRV can provide useful information about autonomic control of the cardiovascular system. In our study the subjects were asked to perform sub-maximal exercise (50% of VO2 max.) to assess their sympathetic activity. When HRV was taken immediately after exercise,while the male HRV values was more than their respective resting HRV though not significant , the female HRV was significantly more than their respective resting HRV; indicating the anticipatory response of exercise, which is mediated through sympathetic activity is more in female. During exercise the heart rate increases, so should the activity of the sympathetic nervous system but in the study we find only a marginal rise in LF/HF ratio. This can be explained on the lines of homeostasis in which the body tries to maintain an equilibrium condition. The sympathetic activity (LF) increases but simultaneously the parasympathetic activity (HF) also increases to maintain equilibrium. This saves the heart from an overdose of sympathetic stimulation, suggesting a more stable autonomic environment for the heart.31The results can also be described as, the study being conducted on sub-maximal exercise the sympathetic nervous system did not reach to its peak values hence though there was increase in LF/HF ratio, there was no significant change in LF/HF ratio, this is on lines of the study conducted by Ana Márciados Santos António et al.32 Several authors have observed that obesity and weight loss affect HRV.33,34,35,36,37 Diet induced weight loss improved baroreceptor sensitivity and metaboreflex in obese subjects. 38,39 However, others40,41,42 have detected no association between HRV and BMI. A study conducted by Breno Quintella Farah, et al. 22 on HRV and its relationship with central and general obesity showed that the body mass index showed no significant correlation with any heart rate variability parameter. Our study agrees with those studies which show no association between HRV and BMI in case of males. In the present study (Table no.7, Fig.5and6)it is seen that there is no significant correlation of both Pre and Post exercise LF/HF ratio with BMI in males. But in our study it shows that with increasing BMI in females the LF/HF ratio decreases in both resting and post-exercise conditions and that association is significant only in pre-exercise condition. It has been documented with various methods in experimental43 and clinical studies 31,44,45,46that parasympathetic activity is decreased in obesity. Katarzyna Piestrzeniewicz et al.47demonstrated a negative correlation between waist circumference and the parameters reflecting parasympathetic activity. Disparate results come from the study by Kim et al.23 who revealed a negative correlation between HRV and waist to hip ratio. In our study (Table no.8, Fig.7 and 8) there is no significant correlation of pre and post exercise LF/HF ratio with Hip ratio in males, but in case of females (Table no.10, Fig.11 and 12) it was found that in post exercise conditions LF/HF ratio has a significant negative correlation with WHR. Physical exertion acutely increases cardiac output and results in a redistribution of blood flow to meet the metabolic demands of active skeletal muscle. After exercise, there is a prompt decrease in cardiac output as physiological activity returns towards resting conditions. Specialized afferent receptors, such as metaboreceptors and baroreceptors, are highly involved with the changes in cardiovascular-autonomic modulation during and after exercise. However, increased intramuscular fat reduces skeletal muscle uptake of glucose and attenuates acidosis during exercise48, and therefore, could lower the activation of the metaboreceptors49. Furthermore, reduced baroreflex sensitivity following brisk walking has been reported in obese compared to lean women50. Consequently, it appears that the afferent feedback mechanisms that normally result in an appropriate autonomic adjustment of the cardiovascular system during and following exercise is impaired with obesity49, which help to explain the results of the current study.
CONCLUSION Obesity is frequently associated with sympathovagal imbalance characterized by depressed parasympathetic tone and increased sympathetic activity. The present work opines that the females have higher parasympathetic activity than males. Observing the association between WHR and BMI with HRV in healthy female persons suggests that ANS dysfunction can be detected early and at relatively young age, therefore providing an opportunity for early intervention. Present study also shows that there is an increase in LF/HF values among males and females after a single bout of sub-maximal exercise though the change is not significant. So, exercise therapy programs of variable intensities may increase HRV in obese individual. Future investigations on larger groups of patients, with HRV in response to stimuli, complex evaluation of ANS including baroreceptor reactivity as well as prospective study with follow-up observation are warranted to thoroughly elucidate the impact and mechanism of adverse effects of obesity on cardiac function. Further research is needed to identify exercise regimen that produces optimal improvement in HRV. It also needs to be evaluated whether exercise interventions are effective in long-term therapies for stimulating favourable ANS alterations.
Acknowledgement: Indian Council of Medical Research.STS2014(REFERENCE ID: 2014-00536)
REFERENCES
Policy for Articles with Open Access
|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 Home
Home