|
Table of Content - Volume 20 Issue 1 -October 2021
Radioiodinated melatonin: Preparation and purification using separation techniques
Lathika1,2, Manupriya B R2, Swaroop K3,4, V B Kadwad5, K Bhasker Shenoy6, H M Somashekarappa7, Shrikant L Patil8*
1,8Department of Physiology, K. S. Hegde Medical Academy, Deralakatte, Mangalore, Karnataka, INDIA. 2,6Department of Applied Zoology, Mangalore University, Mangalore, Karnataka, INDIA. 3Department of Physics, G. M. Institute of Technology, Davangere, Karnataka, INDIA. 4Centre for Application of Radioisotopes and Radiation Technology, Mangalore, Karnataka, INDIA. 5,7Radiopharmaceuticals Programme, Board of Radiation and Isotope Technology (BRIT), Vashi, Navi Mumbai, Maharashtra, INDIA. Email: shrikantlpatil@gmail.com
Abstract Background: Melatonin is a vital hormone secreted mainly by the pineal gland and synthesized in many tissues and cells. The melatonin secretion is stimulated by the dark (night) cycle and inhibited by the bright (day) cycle. It conveys the light-dark cycle to the organism, organizing its seasonal and circadian rhythms and controlling various body functions. The present work aims to develop a tracer using the chloramine‑T method for radioimmunoassay (RIA) procedure and estimate the optimum amount of chloramine‑T required to prepare a stable radioiodinated product with a specific activity. Melatonin was subjected to radioiodination using the chloramine‑T method, with different concentrations of the chloramine‑T solution to achieve a stable radiolabeled molecule/compound. The critically optimized reaction conditions relating to the concentration of chloramine‑T (10 µg) and MBS (20 µg) yielded a stable 125I‑Melatonin with the specific activity of 22.2 MBq/µg corresponding to 0.79 125I atoms per molecule of the peptide. Our study achieved high integrity in preparing melatonin tracer by combining one molecule of oxidant (chloramine‑T). Keywords: Melatonin, Radioiodination, Chloramine-T, 125-I.
INTRODUCTION Melatonin secretion follows a circadian rhythm and is entrained to the light/dark cycle; light suppresses melatonin production. Melatonin is produced and secreted from pinealocytes.1 Light input is transmitted from the photic receptors in the retina through the retinohypothalamic tract to the suprachiasmatic nucleus (SCN), which is located in the anterior part of the hypothalamus and functions as the central circadian pacemaker of the body. The secretion of melatonin is also associated with the reproductive rhythm. In humans, melatonin secretion is inversely correlated with gonadal development; peak melatonin levels fall just before the onset of puberty.2 Melatonin is also involved in immune function. Evidence suggests an immune-enhancing function for melatonin via stimulating natural killer cell activity, cytokine expression regulation, and apoptosis inhibition in immune cells. In support of such a function, high-affinity melatonin receptors have been detected in human T lymphocytes.3 Melatonin’s other functions include free radical scavenging and DNA repair via immune modulation, promoting wound healing and body weight control to the association of environmental cues to biological clock gene expression, and regulating secondary endocrine signalling (e.g., prolactin release, estrogen receptor-mediated signalling). Melatonin not only targets the mammalian skin and hair follicles but is also secreted from these sites, which is known as extra pineal melatonin synthesis. Melatonin acts as a vital skin protectant, and its functions may impact skin biology and pathology.4 The first quantitative estimation of melatonin was done by Mori and Lerner.5 Since the bioassay was too tedious and insensitive for routine use, in the 1970s, methods like gas chromatography-mass spectrometry6 and Radioimmuno assays were developed.7 Chegini et al.8 compared Enzyme-Linked Immunosorbent Assay (ELISA) for melatonin with radioimmunoassay, where radioimmunoassay showed more sensitivity and the detection range of melatonin. However, ELISA did not cover the physiological daytime melatonin concentration in humans. The estimation of plasma and serum melatonin levels in mice by ELISA or HPLC-technique previously failed due to the required use of a high sample volume and the low sensitivity. Most of the RIAs hitherto developed for melatonin use tritiated melatonin as a tracer. We have carried out the iodination experiments of a melatonin derivative that melatonin itself can be iodinated. This work has highlighted the preparation of [125I] melatonin and its application in the indigenous development RIA of melatonin of human serum and plasma. The distinct advantage of utilising [125I] melatonin as a tracer is that the costly and cumbersome scintillation counting can be avoided.
MATERIALS AND METHODS Biochemicals Synthetic human melatonin (Molecular weight 238.278 g/mol) with a purity of >98% from Sigma-Aldrich Co. LLC, melatonin, Sephadex G-75 and Bovine Serum Albumin (BSA; RIA Grade) were purchased from Sigma Chemical Company, USA. Polyclonal antibody to melatonin raised in rabbits was purchased from Abbexa Ltd, UK. Bovine serum was from Himedia, India. Carrier free 125I as Sodium Iodide (Specific activity ~17mCi/µg, radioactive concentration 100mCi/mL) was obtained from BARC, India. Whatman 3mm chromatography paper was purchased from Whatman Ltd, England. Magnetizable cellulose particles were prepared in-house at BRIT Laboratories, Mumbai, India (Indian Patent No: 193445). All the other chemicals were of highest purity available from local manufactures from India. Equipment used The major equipment used in this study includes a single well manual gamma counter (ECIL, India), a multi-well gamma counter (STRATEC, Germany including data processing), RIA Centriguge (REMI R-23, India) and CM 101 cyclo mixer (REMI, India). All the graphs and analyses were carried out using OriginPro 8 SR0 (v8.0724 (B724), www.OriginLab.com, USA) Iodination of melatonin using Chloramine-T method: Reaction Mixture: 10µL of melatonin and 80µL of 0.05M PO4 Buffer is taken in a tube. 5mCi of 125I and 10µL of Chloramine-T was added, the mixture was allowed to react for a minute. After a minute, 500µL of 0.05M PO4 Buffer, 25µL of SMB, 50µL of KI were added. Half of the mixture was added to the column, and the remaining were used for TLC for purification. Few drops were subjected to electrophoresis. Paper electrophoresis: Electrophoresis tank was half-filled with 0.025M PO4 Buffer. Take around 34cm of Whatmann paper 3MM and mark for each cm till 30cm. Saturate the strip in the buffer for 15 min. Spot a drop of KI and then reaction mixture on the strip and run for 50min at 240 volts. After 50min, dry the strips cut them into 1cm and take the counts per minute (CPM) using an automatic gamma counter. The same procedure is followed for purified and pooled fractions. Calculation: The yield of reaction mixture and radiochemical purity of tracer can be calculated using the following formula %yield / RCP= Counts of peak / total counts X 100 Column Chromatography: 40 tubes were kept ready to collect the fractions eluted from the column by adding 1mL of 1% BSA in each tube and marking the tube for 2cm. As soon as the reaction mixture was added to the column, 1mL of fractions were collected in the tubes at 8-12 drops/min flow rate. The counts were taken in a manual counting machine for 10 sec. The collection was stopped once the counts decreased after two peaks. The fractions were pooled depending on their counts, and a tracer was selected. Thin Layer Chromatography: Preparative chromatography plate was marked for spotting the sample (1cm from the bottom) and 2/3rd of the plate to stop the procedure. The chromatography chamber is sealed and saturated with ethyl acetate for an hour. The reaction mixture is spotted on the plate with continuous drying, and then it was allowed to run till the solution reached 3/4th of the plate. Then the plate was taken out, dried, and bands were seen under a UV scanner or by developing an X-ray. The bands obtained from X-ray development showed the location of the tracer on the TLC plate. The TLC plate is marked, and silica was scraped and collected in a tube of both band and spotted area. Counts were taken. Then they were purified by methanol thrice; after each wash, CPM was counted. The 1st two washes had the highest CPM, which decreased during the third wash. So, the washing process stopped. The 1st two washed samples were pooled and stored. Sephadex Gel Filtration: The purification of the reaction mixture was performed by a Sephadex gel filtration method on Sephadex G 100 column (1 X 25 cm). The whole reaction mixture was applied to the column, pre-calibrated with buffer pH 8.2 and bovine serum albumin.
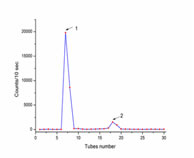
RESULTS AND DISCUSSIONS In the present study, the iodination efficiency obtained is 11%, more significant than the yield (4%) reported by Vakkuri et al.9 The radioiodination procedures were similar in both the studies, but the pH used in the current study was 7, whereas the latter study used pH -6. Although pH from 6 to 8 is used for chloramine-T oxidation methods, it is suggested that pH- 7 is suitable for chloramine-T oxidation effectively. For melatonin RIA, Iodogen, and Bolton-Hunter reagent have been previously used to prepare radioiodinated tracer, but these reagents are expensive compared to Chloramine-T.10-13 The rapid removal of the unchanged 125I by gel filtration minimizes the external radiation of melatonin, and it requires a small quantity of melatonin to iodinate, and good yield was recovered in an hour, and similar results were seen in separating iodinated insulin. The counts obtained from gel filtration gave two peaks, iodide and iodomelatonin peak (Figure 1).
Figure 1: Paper Electrophoresis pattern, 1st peak is iodide, 2nd peak is iodinated melatonin.
The paper electrophoresis confirmed that the free iodide gets eluted first, followed by iodomelatonin and the RCP of iodomelatonin was 94.31%, and it was stable till two months. The X-ray film developed for the TLC plate showed the iodomelatonin band, and the Rf value of iodomelatonin obtained was 0.38, and the RCP obtained was 15%. The comparative study between TLC and HPLC methods used to purify 2-[125I] Iodomelatonin showed that the yield obtained by TLC was about 15-30%, and by HPLC was 30-60%. It is also concluded that to get a high yield from TLC separation, the reaction mixture should contain a high amount of protein to be iodinated. Since, in the present study, a small amount of melatonin was used, light iodomelatonin bands were seen in the X-ray film (Figure 2). The X-ray film development reduced the time and extra work of scraping and counting the silica of the whole TLC plate, which was previous researchers practised. The prepared 125I-melatonin, when allowed to bind to melatonin polyclonal antibody, gave 28% binding and confirmed that the tracer is 125I-melatonin. Figure 2: X-ray copy of TLC, 1- iodinated melatonin band, 2- spotted area. Standardization of radiolabelling method is done by changing the concentration of the reducing agent and other chemicals. The radiolabelling efficiency is confirmed by the paper chromatography and the thin layer chromatography (TLC) techniques (Table 1). So, the present study concludes that melatonin and its radiolabelled form are more efficient with the Chloramine-T method.
Table 1: Radiolabelling Efficiency of melatonin with 125I
All the values of radiochemical purity were above 93% (Table 2). According to standard specifications, the melatonin with 125I radiochemistry purity must be superior to 90%. The results presented in Table 2 showed that the differences in the radiochemical purity determined by Paper Chromatography and TLC methods were not statistically significant. However, both methods can be used in the quality control of radiopharmaceuticals.
Table 2: Comparison of % radiochemical purity of melatonin with 125I by Paper Chromatography and TLC methods
Data presented as ±SEM One-tenth of the filtrate was collected in each tube, and the radioactivities were counted with a well-type gamma scintillation counter (Table 3). The approximate labelling yield was calculated in connection with the radioactivity absorbed by iodine beads.14 Table 3: Comparison of radioactivity yield of melatonin with 125I
Data presented as ±SEM The average labelling yield was 87.5%. The impact of the increasing amount of Chloramine T solution and the influence of slight prolonging of reaction time on the labelling yield were not statistically correlated.
CONCLUSIONS The reagent mixture concentration of 10 µg chloramine‑T and 20 µg MBS and 20 µg chloramine‑T and 40 µg of MBS produced a radiolabeled compound with a yield of 85% and 88%, respectively. Among these, 10 μg of chloramine-T and 20 μg of MBS were selected to carry out radioiodination. This concentration combination offers a radiolabeled product with good radioiodination yield and minimum damage to the protein. In this study, it was found that two molecules of reductant are essential to hinder the action of one molecule of the oxidant. 125I‑melatonin can be used for the routine preparation of tracer required for the regular production of melatonin RIA kits. Our study showed that there were no significant differences in the radiochemical purity determined by Paper Chromatography and TLC. The Sephadex gel filtration method is much superior to any other separation techniques used for radiolabelling studies. We have also assessed various concentrations of both melatonin and 125I in order to achieve easy labelling with high efficiency. Acknowledgements: We acknowledge the financial support by Board of Research in Nuclear Sciences (BRNS), Mumbai through Centre for Application of Radioisotopes and Radiation Technology (CARRT), Mangalore University, Manglaore. The authors are grateful to Mrs. Jayula Sarnaik, Mrs. Rani G. and Mrs. Shalaka Paradkar of the Radiopharmaceuticals Programme, Board of Radiation and Isotope Technology (BRIT), Vashi, Navi Mumbai, Maharashtra, India for providing the necessary laboratory and radiolabelling facilities.
REFERENCES
Policy for Articles with Open Access
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 Home
Home