Official Journals By StatPerson Publication
|
Table of Content - Volume 3 Issue 3 - September 2017
Effect of circadian rhythms and gender in patients with ST-Segment elevated myocardial infarction
Sunita Handergulle1, Sidheshwar V Birajdar2, Ashishkumar Jain3*
{1Professor and HOD, Department of Physiology} {2Professor and HOD, 3Sr. Resident, Department of Medicine} SRTR Government Medical College, Ambajogai, Maharashtra, INDIA. Email: dr.ashish31.jain@gmail.com
Abstract Background and Aim: The circadian rhythm, body’s biological clock, is known to influence a number of physiological and pathological processes in the body, including the development of acute myocardial infarction. Therefore, in our study, the role of circadian rhythm in patients with ST-Segment Elevated Myocardial Infarction (STEMI) has been studied. Methods: In this hospital-based, cross-sectional study, 200 patients were chosen as per the selection criteria from among the acute myocardial infraction (MI) patients admitted in the ICU, Department of Medicine, SRTR GMC, Maharashtra. Results: Of the 200 subjects, 164 (82%) survived MI while 36 (18%) developed complications. Maximum MI occurred early in the morning. A progressive increase was seen in the incidence of anterior wall myocardial infarction as age advances. There is a statistically significant higher occurrence of systemic hypertension in female subjects compared to the incidence of other comorbid illnesses among males and females. Conclusion: Occurrence of STEMI shows the first peak between 6 AM and 12 PM and the second peak between 12 PM to 6 PM. There is a statistically significant higher occurrence of systemic hypertension in female subjects compared to the incidence of other comorbid illnesses among males and females. There is no significant correlation between the time of onset of symptoms and complications in any particular gender. Mortality was higher in males and the deaths in each period were proportionate irrespective of the circadian pattern. Key Words: Anterior wall myocardial infarction, STEMI, circadian rhythm, gender.
Circadian clocks have been identified in every mammalian cell investigated to date.1 There are known diurnal variations in the physiological functioning of cardiovascular system.2 Historically, diurnal variations in blood pressure, heart rate and cardiac output, in addition to major cardiovascular events like myocardial infarction and sudden death, have been attributed primarily to the occurrence of circadian changes in the autonomicnervous system – that is, sudden increase in sympatheticactivity.3,4 Some authors have identified anearly morning to mid-morning peak in mortality rates among patients with acute myocardial infarction.5 Multiple biological factors have been postulated as contributing factors to the effect of the circadian rhythms, including cyclic patterns in heart rate, QT interval, hemostasis, platelet aggregation, lipoprotein levels, hormonal levels and sympathetic tone.6-8 In addition, there are extrinsic environmental factors, patient related factors, that also exhibit circadian patterns, such as patient’s perception of symptoms, willingness to go to the hospital and choice and timeliness of reperfusion therapies during regular working hours of hospitals versus the emergency hours of the day.9 The effect of circadian rhythm and gender of a patient, on acute myocardialinfarction has been the subject of considerable scientific interest and controversy since a few years now. This study was sought to evaluate the role of circadian rhythm and gender in patients with ST-Segment Elevated Myocardial Infarction (STEMI).
MATERIALS AND METHODS The present study was done on 200 patients with the first attack of ST segment elevated myocardial infarction (STEMI)(164 males and 36 females). The patients to be included were chosen as per the inclusion and exclusion criteria from among the acute MI patients admitted in the Intensive Care Unit (ICU) of Department of Medicine, SRTR GMC, Maharashtra, from May 2017 to July2017.Patients aged 30 years and above with the first attack of acute STEMI presenting at the ICU were included in the study. Patients with uncontrolled diabetes mellitus or systemic hypertension or with any other associated heart disease such as valvular heart disease or cardiomyopathy or blocks, preceding angina, were excluded from the study. After getting approval of the Institutional Ethics Committee (IEC), informed consent was obtained from the study participants before collecting the data. Details of the demographic and clinical variables were recorded in the case record form and the time of onset of symptoms and occurrence of complications were obtained from the ICU medical history. Statistical analysis of data: Data collected from the patients was entered in the case record form. SPSS of Windows version 10, EpiInfo Version1.1 was used for data analysis. Student’s t-test was applied to ensure the comparability of data. Association between variables was assessed using the Chi-square test. ST-segment Elevated Myocardial Infarction was defined by: Typical chest pain of ≥30 min and significant ST-segment elevation (≥0.1 mV or ≥0.2 mVon ≥2 adjacent limb or precordial leads (Minnesotacode), respectively, or new left bundle-branch block and confirmed by a rise in biomarkers more than twice the upper limit of normal. Time of onset of symptoms and of complications was first divided into four 6 hourperiods.10 Accordingly, the following periods were chosen - Period1: 12 am – 6:00 am, Period 2: 6:00 am –12 pm, Period 3: 12 pm –6 PM, Period 4: 6 pm–12 am. RESULTS Of the 200 subjects, 164 (82%) were males and 36 (18%) were females, their age ranging from 30 to 82 years. The mean age was 56 years. The subjects were classified according to their age and gender into four groups of ≤40 years, 41–50 years,51–60 years, and ≥61 years. There was a progressive increase in the incidence of STEMI as age advances. This age wise and gender-wise distribution is shown in Table 1.10.5% of the study subjects were young (≤40 years of age) and all of them were males. Males were maximally affected in the age group of > 51 years followed by age groups 41–50 years and least in < 40 years age group. About two-thirds of affected females were above 60 years of age. A maximum number of females with STEMI were in the age group ≥61years followed by 51–60 years age group (25 [69.45%] and 8 [22.22%]) respectively.
Table 1: Age-wise and gender-wise distribution of study subjects
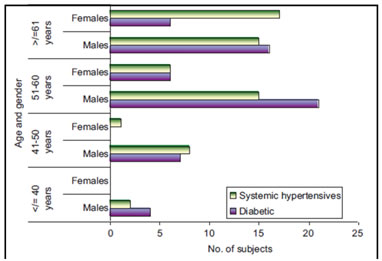
On studying the risk factors, a majority of the males – 72 (44%) patients, were smokers. This had a highly significant P value (male–female difference: 0.0001). 24(15%) males were alcoholics. There were no alcoholics/smokers among females. 80% of diabetics and 62.5% of systemic hypertensives, respectively were males. About 87.5% of subjects with family history of CAD were also males. In comparison, females constituted 20% of diabetics, 37.5% of systemic hypertensives, and 12.5% with a family history of CAD. The presence of diabetes mellitus was found to be more frequentin male STEMI patients >50 years of age, as shown in Table 2.However, the association of age and gender with the occurrence of diabetes mellitus was not significant in any age group. These results are depicted in Figure 1.There is a statistically significant higher occurrence of systemic hypertension in female subjects compared to the incidence of other comorbid illnesses among males and females (P value of male–female difference: 0.0001).These findings are recorded in Table 2. Association of age and gender with comorbid illnesses was studied.27 (45%) subjects (both males and females)in the age group 51–60 years and 22 (29%) subjects ≥61years showed statistically significant occurrence of systemic hypertension with P value of 0.018 and 0.001 in the two age groups, respectively. STEMI was more frequent in females of 51–60 years of age with systemic hypertension (75% female vs. 29% male) and in females≥61 years of age (68% female vs. 29% male). Systemic hypertension was the most frequent comorbid illness more significantly associated with females, 24 (65%) and the P value of male–female difference was 0.0001(Figure 1). Table 2: Frequency of risk factors among study subjects
Figure 1: Age and gender-wise distribution of comorbid illnesses
The occurrence of anterior wall STEMI followed a circadian pattern with the first early morning peak between 6 AM and 12 Noon with 75 (37.5%) cases and the second peak from 12 Noon to 6 PM with 53 (26.5%) arrhythmias being the most frequent complication in all the periods. Of the200 subjects, 166 (83%) survived MI while 34 (17%) developed further complications. The occurrence of the complications was analysed further. Arrhythmias were the most frequent complication seen in 7 (35%) out of 20 subjects in the12 Noon–6 PM quarter. Among the 4 subjects who developed left ventricular failure, the majority 3 (50%) subjects, had symptom onset in the 6 AM–12 Noon period. Mortality was maximum in the period 12 Midnight–6 AM for 4 (40%) out of 10 subjects. Application of statistic analytic tests reveals a non-significant Chi-square test, P = 0.363. These results are shown in Table 3. Table 3: The time of onset of symptoms and the complications developed during each at time period
DISCUSSION Circadian variation in the levels of cortisol, circulating epinephrine levels, coronary flow, blood viscosity, endogenous thrombolytic activity, and platelet aggregability is a well-known phenomenon. Our study demonstrates the early morning peak in MI from 6 AM to12 Noon quarter (37.5%) which correlates with western literature and could be due to the increase in physical and mental stress after waking.11,12 Sympathetic activity increases after waking and plasma concentrations of catecholamine also increase. Cortisol concentration peaksearly in the morning, generally before waking, and it remains high but falls during the period after awakening.13 Systemic arterial pressure14 coronary vascular tone15 and platelet aggregability16 also increase in the earlymorning. Although fibrinolytic activity is low during sleep and still reduced soon after awakening, it rises during this time.17 The second peak from 12 Noon to 6 PM quarter(26.5%) observed in our study is similar to the recent observations made by Sari et al. and Misiriya et al.18,19 The exact reason for this phenomenon remains obscure though genetic variations of the study population,18 and a prothrombotic state during physical inactivity after lunch20 may be the possible explanations.18 There is evidence of differing circadian patterns of symptom for the onset in subgroups of patients with acute MI as inpatients with a history of congestive heart failure, or with non-Q wave infarction, there was a pronounced peak only in the evening.21,22 To study the circadian pattern further, we assessed some socio-demographic parameters such as age, gender, habits, and comorbid illnesses. The mean ageat presentation of patients with STEMI was 57.41 years in this study, which is comparable to observations of CREATE Registry (57.5 years).23 Male preponderance was observed in the patients with STEMI at all age-groups and the sex ratios observed in both the younger and older age-groups were comparable to the sex ratios observed in another series reported from North India.24 In our study population, habits such as smoking and alcoholism were adopted only by males. Behavioral influences, such as mental activity and emotionalstate,25 and lifestyle factors, such as smoking cigarettes and drinking alcohol, have been shown to affect the natural rhythm of blood pressure (BP).26 Smoking can result in coronary atherosclerosis leading to increased risk of myocardial infarction. By increasing myocardialoxygen demand and decreasing oxygen supply, myocardial ischemia is aggravated. Smoking also can result in systemic hypertension which if sustained, leads to left ventricular hypertrophy, and is thus a major risk factor for ischemic heart disease. A maximum of oneor two alcoholic drinks per day over long periods may decrease the risk of cardiovascular death; perhaps through an increase in high-density lipoprotein cholesterol or changes in clotting mechanism. However, heavy drinking is an important contributor to mild to moderate systemic hypertension that could lead to ischemic heart disease. Disruption of the circadian clock can cause severe disturbances in our body’s endocrine rhythms and the impact of comorbid endocrine disorders such as diabetes mellitus on the morning peak incidence of acute myocardial infarction (AMI) has been reported.27 Some authors have reported that the early morning peak incidence of AMI is attenuated in patients with diabetes28 while others have observed a similar peak incidence of AMI onset in the sleep-to-wake period.29 In addition to diabetes, the glucose levels at the time of AMI onset have been associated with poor outcomes in patients with diabetes.30 Even though the levels of blood glucose are also regulated by the circadianrhythm, the 24-h glucose and insulin secretion raterhythms are only partially adapted in permanent night workers31 and essentially retain their circadian pattern. Increased incidence of large vessel atherosclerosis and myocardial infarction is seen in diabetic patients. Further, their myocardial infarction tends to be larger in size and is more likely to result in complications such as heart failure, shock, and death. BP rises sharply in the morning in response to the activation of the sympathetic nervous system when one awakens.26,32 This early morning surge is associated with other important hemodynamic and neurohormonal changes, including increase in heart rate, vascular tone, and blood viscosity and decrease in vagal activity.32 Early morning BP surge is associated with an increase in the incidence of cardiovascular events such as MI.10 Our study shows a highly statistically significant (P value of male–female difference was 0.0001) association of STEMI with systemic hypertension in female subjects. STEMI was more frequent in female hypertensives who are >50 years of age. Manfredini et al.9 have studied that the time of symptomon set could potentially influence mortality in acute MI since little is known about the possible influence of circadian pattern on prognosis in MI. It was seen that not only the frequency but also the mortality in acute MI could be increased in the morning hours. This correlates with our finding of highest mortality of 40% in the period12 Midnight–6 AM. Gender-wise difference could not beassessed as this complication affected only males (in our study) and 6 (60%) of them were >50 years of age, but the number of deaths in each period was proportion ateirespective of the circadian pattern. However, mortality ate was lesser among our STEMI cases compared to the mortality rate observed among cases from the CREATE Registry 12 (5% vs. 8.6%),23,33 which could be due to improved hospital care. LIMITATIONS OF THE STUDY: The number of females recruited in the study was comparatively less, and hence, the non-significant statistical test results may be attributed to this less number of subjects. A higher sample size could increase the power of the study.
CONCLUSION The occurrence of STEMI showed the first early morning peak between 6 AM and 12 Noon and the second peak from 12 Noon to 6 PM with arrhythmias being the most frequent complication in all the periods. There was a progressive increase in the incidence of STEMI within creasing age, and younger males and elderly females were the most affected. There is a statistically significant higher occurrence of systemic hypertension in female subjects compared to the incidence of other comorbid illnesses among males and females. Our study reports that males, especially those >50 years of age, have higher mortality rates. Diabetic patients with STEMI have greater risk of death. Females, elderly individuals, and those with systemic hypertension and diabetes mellitus should be managed more carefully to reduce the mortality rates.
REFERENCES
|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 Home
Home