Official Journals By StatPerson Publication
|
Table of Content - Volume 7 Issue 1 - July 2018
Study of coagulation profile in IHD patients
Aiesha Durrebar Younus Khan1, Ashwini D Pujari2*
1Assistant Professor, Department of Physiology, Shadan Institute of Medical Sciences, Himayatsagar Road, Rangareddy, INDIA. 2Professor, Department of Physiology, Mahadevappa Rampure Medical College, Sedam Road, Mahadevappa Marg, Kalaburagi-585105 Karnataka, INDIA. Email: drashwinidp@gmail.com
Abstract Background: Epicardial coronary arteries are the major site of atherosclerotic disease. A thrombus composed of platelet aggregates and fibrin strands traps red blood cells and can reduce coronary blood flow, leading to the clinical manifestations of ischaemic heart disease (IHD). A positive association of coagulation factors has been implicated with risk of IHD. This study was under taken to assess the association of coagulation factors in IHD patients by comparing them to normal subjects. Methods: This case control study includes fifty IHD male patients aged 30-50 years as cases and fifty apparently healthy age and sex matched male subjects as controls. Venous blood samples were collected from the subjects which were analyzed for coagulation parameters such as prothrombin time (PT), activated partial thromboplastin time (aPTT) and fibrinogen levels. Result: The PT and aPTT in cases were significantly lower and fibrinogen levels were higher in cases than in controls. Conclusion: Shortened levels of PT and aPTT are seen in IHD patients than compared to normal subjects and hence these tests prove as reliable diagnostic tools for IHD. Higher fibrinogen levels, by means of increasing viscosity, also seems to be an independent risk factor for IHD. Key Words: Activated Partial Thromboplastin time, Fibrinogen, Ischemic Heart Disease, Prothrombin time.
Cardiovascular diseases (CVD) manifest in the form of ischaemic heart disease (IHD), stroke, congestive heart failure, cardiomyopathy and arrhythmias. These diseases have emerged as a major health problem worldwide, with IHD being the responsible for one third of all global death.1 A number of studies have demonstrated the role of thrombus formation in the pathogenesis of IHD. There are two main types of measurements related to haemostatic disturbances in the investigations of the prothrombotic state in cardiovascular disease: measurement of levels of haemostatic factors and measurement of activation products of hemostasis. Measurement of levels of hemostatic factors include fibrinogen, factor VIII, factor VII and vWF.2 A tendency to thrombosis may be indicated by elevated levels of fibrinogen and factor VII clotting activity (FVIIC).3 Initially believed to measure Prothrombin (Factor II) and hence named so, Prothrombin Time (PT) was subsequently found to be sensitive to abnormalities of factors VII, X, V, II and fibrinogen. PT thus measures the activity of the extrinsic pathway which is dependent on the functional activity of factors VII, X, V and II.4 Studies also reveal contributions of the intrinsic pathway of blood coagulation to thrombogenecity.5 This pathway can be analyzed by measuring activated partial thromboplastin time (aPTT).4 aPTT test was originally performed to screen the function of coagulation system, mainly for the intrinsic pathway factors: factor XII, XI, IX and VIII.4 PT and aPTT are important basic investigations in clinical laboratories and can be used to assess the risk of clotting complications. The present study is carried out to assess the coagulation profile i.e. PT, aPTT and fibrinogen levels in IHD patients and to compare the findings with normal controls.
MATERIALS AND METHODS Study Design: The study was conducted in the Department of Medicine, Basaveshwar General and Teaching Hospital, Kalaburagi Study Group: Included fifty newly presenting IHD male cases aged between 30-50 years, diagnosed by the physician on the basis of history, electrocardiographic findings and other clinical evidences. Control Group: Fifty healthy male individuals accompanying the patients were age and sex matched to the study group subjects and were included as subjects for the control group. Exclusion Criteria
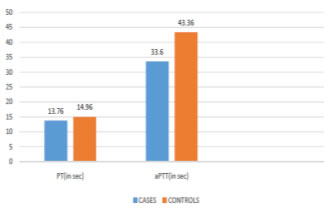
Method of collection of data After obtaining ethical clearance from the institution and informed written consent from each subject a prepared questionnaire was given to each subject to answer. The preformed questionnaire included detailed history of sociodemographic data, history of presenting illness, pastand family history including history of hypertension, diabetes mellitus, ischaemicheart disease and thyroid disorders, personal history including type of diet and anyhistory of addictions such as smoking history and alcohol intake were noted. Thorough general physical and systemic examinations were done. Venous blood samples were collected from each subject for analysis of blood coagulation tests i.e. prothrombin time, activated partial thromboplastin time and fibrinogen level as well as for determination of the blood group. Determination of blood groups was done using slide agglutination technique. Blood coagulation tests were carried out in the Biochemistry laboratory of Basaveshwar Teaching and General Hospital on Sysmex CA-50 (Code- 461-2035-2 Sysmex Corporation Kobe, Japan) Automated Blood Coagulation Analyzer. All data is expressed as mean± standard deviation. Students unpaired t test and One way ANOVA were applied to analyze the data. The p value <0.05 was taken as statistically significant and p value< 0.01 was taken as highly statistically significant. On comparing the coagulation profile amongst cases and control it is observed that the prothrombin time is significantly lower in cases than compared to the controls. Also the activated partial thromboplastin time is shortened in cases than in controls with the difference being highly statistically significant. Although the fibrinogen level is more in cases than in the controls, it is not statistically significant.
Table 1: Comparison of Coagulation Profile between Cases and Controls
*p value <0.05-statically significant, **p value <0.01-highly statically significant
Figure 1: Comparison of PT and aPTT between cases and controls
Fibrinogen and IHD: Our study shows that the fibrinogen level amongst cases was higher than the control group which is consistent with several other studies.7,8 High fibrinogen levels may promote cardiovascular disease through arterial wall infiltration and effects on blood viscosity, platelet aggregation, and fibrin formation.9 Relation of Coagulation Factors with IHD: On comparing the coagulation parameters between the cases and controls it is was seen that the PT is significantly lower in cases than in the controls with a p valueless than 0.05. PT gives an indication of the concentration of prothrombin in blood and the shortness of the time is determined mainly by the prothrombin concentration.10PT hasnow been found to measure the activity of the extrinsic pathway which is dependenton the functional activity of factors VII, X, V and II.4 A prolonged prothrombin timeindicates a deficiency in any of factors VII, X, V, prothrombin, or fibrinogen. On the other hand a decreased prothrombin time is responsible for increased clottingtendency.4 Factor VII and IHD: The level of FVIIC is influenced by Arg353 allele of the factor VII gene. Andhence the FVIIC is more in those individuals in whom the Arg353 allele is expressed on the factor VII gene. Those who express polymorphism of factor VII genes, show lower levels of FVIIC and lower levels of factor VII antigen and thereby are provided protection from MI.11 The first NPHS (Northwick Park Heart Study) on 1511 men has shown that high levels of factor VII coagulant activity is associated with increased risk of an IHD event. The elevations of one standard deviation in FVIIC increases the risk of an episode of IHD by 62%.3 An Italian case-control study reported that FVIIC and factor VII antigen are higher in cases with familial MI than in controls.11 In contradiction to the above studies, findings from the ARIC (Atherosclerosis Risk in Communities) study did not find factor VII associated with CHD events.12 However to justify these ambiguous findings, Ralf Junker et al have concluded that higher levels of FVIIC is associated with increase in the in number of CHD events only in the presence of additional cardiovascular risk factors such as smoking, family history, high fibrinogen levels, high levels of total cholesterol, low density lipoproteins (LDL) and triglycerides and a low level of high density lipoprotein (HDL) cholesterol.13 More prospective studies are needed to clarify the association between factor VII and CHD. In our study we indirectly measured the coagulation activity of factor VII by performing PT in our study subjects. Individual factor assay was not done in our study and hence more elaborate studies involving individual factor assays are required to substantiate the involvement of increase in coagulant activity of individual factors with that of IHD. Further more extensive genetic research is also simultaneously needed to throw light on the cause of increased coagulant activity of individual clotting factors of the extrinsic system which would indirectly affect the PT. Our study reveals that the cases have highly significantly shortened aPTT than compared to the individuals of the controls. aPTT measures the function of factors VIII. IX. XI and XII. Factor VIII and IHD: Taking account of evidence that haemophiliacs seem to experience less IHD than expected, high factor VIII levels may contribute to the incidence of IHD byincreasing thrombogenic potential.14 Abnormally shortened aPTT was reported to be associated with elevatedplasma level of FVIII.15 In a study by Abdullah WZ.et al. in 2010, it was observed that there was a significant negative correlation between aPTT and FVIII.16 Apart from factor VIII, aPTT also measures the activity of other factors involved in the intrinsic pathway. Factor XI and IHD: Factor XI, plasma thromboplastin antecedent (PTA), is the second factor that is sequentially activated in the intrinsic cascade by activated factor XII. It is involved in normal thrombin and fibrin formation. Elevated levels of factor XI have been found to be associated with an increased risk of thrombosis.17 Studies such as the Study of Myocardial Infarction Leiden (SMILE) and a Swiss case-control study have found that a higher coagulant activity of factor XIincreases the risk of MI.18 However, the results of a recently published case-control study that included young women did not find a difference in level of factor XI between 200 women witha first MI and control subjects.19 Factor IX and IHD: Sequential to the activation of factor XI in the intrinsic cascade, factor IX i.e. the Christmas factor gets activated. Increased levels of factor IX has been associated with the risk of MI. The SMILE study has also revealed that Factor IX increases the risk of MI, even more strongly among young men.20 Factor XII and IHD: Activation of Factor XII initiates the intrinsic pathway of coagulation and viasequential activation of coagulation factors XI, IX, VIII and X, thrombin is generated, which converts fibrinogen into fibrin.[10]Factor XII is essential for contact activationand the aPTT.4 Factor XII deficiency does not produce any bleeding manifestations and is themost common cause of an isolated prolongation of the aPTT in a non-bleeding individual. For this reason most patients with factor XII deficiency are detected only during a routine preoperative coagulation study.21 However, there is an increased incidence of serious thromboembolic problemsin patients with hereditary factor XII deficiency.21 Lower levels of factor XII result inenhanced thrombus formation by decreased fibrinolysis or effects on inflammation and complement activation.20 There is a high degree of homology between the proteins of the coagulationsystem and those of the fibrinolytic system: Factor XII is homologous to tPA and the two main substrates of activated Factor XII, prekallikerin and Factor XI, arehomologous to plasminogen. Thus activated factor XII binds to plasminogen converting it into plasmin and thus Factor XII participates in the fibrinolytic system.22 The role of factor XII played in the coagulation and fibrinolytic systems requires further research in order to determine its associated risk with IHD.
CONCLUSION It can be concluded that higher serum fibrinogen levels, a shortened PT, which may be due to elevated levels of either factor VII, X, V or II or a shortened aPTT, which may be due to elevated levels of either factor VIII, IX or XI could be used as a screening tool for hypercoagulable state during acute thrombotic episodes.
REFERENCES
|
|
 Home
Home